Video
Gerald Quigley: Mitochondria, CoQ10, and UbiquinolCoenzyme Q and mitochondrial function -
Only a small amount comes from dietary intake approximately 5 mg. The biosynthesis limitation of CoQ10 may also result from genetic mutations, pharmaceuticals, or illness. CoQ10 deficiency has been correlated to the pathogenesis of various disorders, potentially due to its connection with mitochondrial dysfunction.
Mitochondrial dysfunction can result from numerous diseases and may impair ATP production and increase oxidative stress, inflammation, and endothelial dysfunction in the body.
CoQ10 exhibits anti-inflammatory and antioxidative properties that can help to improve mitochondrial function. The positive impact of CoQ10 on mitochondrial health has been researched in patients with diabetic retinopathy , fibromyalgia , heart failure , cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, non-alcoholic fatty liver disease, and neurodegenerative diseases.
The authors concluded CoQ10 to be a safe, well-tolerated supplement that may reduce adverse cardiovascular event risk. The treatment group had a fourfold greater CoQ10 concentration than the placebo group and a statistically significant increase in mitochondrial efficiency and mitochondrial protection from oxidative stress at the time of surgery.
Although CoQ10 is technically not a vitamin, it has been described as vitamin-like due to its essential function in every living cell of the body. The role of CoQ10 for mitochondrial function may result in clinically relevant benefits to various conditions and an overall general well-being.
By Danielle Moyer, MS, CNS, LDN. Designs for Health has been dedicated to being the most trusted source for superior quality, science-based nutritional products for nearly three decades. The blogs we publish here cover a range of topics, including new and original approaches to diet and healthcare, analyses of the latest cutting-edge research, deep-dives into specific nutrients, botanicals, nutraceuticals and more, all fully referenced for those who want to dig deeper into the primary literature.
CoQ10 Helps Promote Mitochondrial Function. August 17, - facebook twitter linkedin. Load more. Search Designs for Health Search form Search. LATEST POSTS. Micronutrients to Promote Endothelial Integrity and Healthy Inflammatory Status.
New Review Investigates the Efficacy of Different Probiotics in IBS. CoQ 10 supplementation for the treatment of any disease should be questioned.
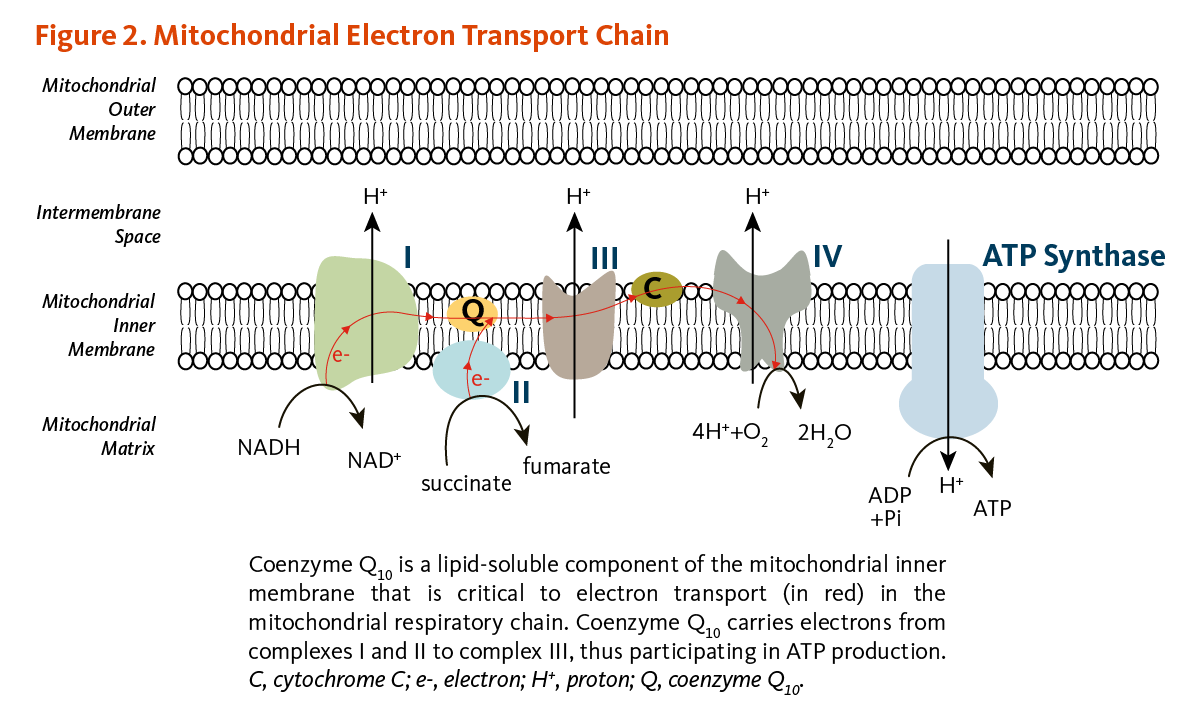
Introduction Coenzyme Q 10 CoQ 10 , also known as ubiquinone UQ 10 , is composed of a redox active aromatic ring and a ten-repeat long polyprenyl sidechain.
Figure 1. CoQ 10 in the mitochondria, pathology of CoQ 10 deficiency and oral supplementation. MATERIALS AND METHODS Search strategy and selection criteria A literature search was performed in PubMed for studies that described PCoQD patients, up until May 01, Figure 2. Flow diagram for identification and selection of primary CoQ 10 deficiency patients.
Data analysis We synthesized data using tabulations that include narrative summaries. RESULTS The literature search yielded 78 published studies, from which a total of patients with PCoQD were identified.
View this table: View inline View popup Download powerpoint. Table 1. Primary CoQ 10 deficiency patients reported in the literature. Table 2. Reported partial effects of CoQ 10 treatment in primary CoQ deficiency patients.
Table 3. Therapeutic efficacy of CoQ 10 suggested by the effects of treatment interruptions. Figure 3. The violin plots of CoQ 10 treatment dose and duration.
Data Availability All data produced in the present work are contained in the manuscript. FUNDING Research in the laboratory of SH is funded by a Foundation grant from the Canadian Institutes of Health Research: FDN Table S2: Cases excluded from the final analysis and reasons for their exclusion.
Table S4: Patient cases classified as not responding to CoQ 10 treatment. S1: The violin plot of total CoQ 10 amounts taken. REFERENCE 1. and S. Hekimi , Understanding Ubiquinone. Trends Cell Biol , OpenUrl CrossRef PubMed. Quinzii , C. Front Physiol , Lenaz , G. and M. Genova , Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject.
OpenUrl CrossRef PubMed Web of Science. Crane , F. J Am Coll Nutr , Morre , D. and D. Morre , Non-mitochondrial coenzyme Q. Biofactors , Bentinger , M. Brismar , and G. Dallner , The antioxidant role of coenzyme Q. Mitochondrion , S41 — Tran , U. and C.
Clarke , Endogenous synthesis of coenzyme Q in eukaryotes. S62 — Wang , Y. Hekimi , Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol , Stefely , J. Pagliarini , Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem Sci , Hekimi , The Complexity of Making Ubiquinone.
Trends Endocrinol Metab , Tsui , H. Clarke , Ubiquinone Biosynthetic Complexes in Prokaryotes and Eukaryotes. Cell Chem Biol , Ogasahara , S. Proc Natl Acad Sci U S A , Hughes , B. Harrison , and S. Hekimi , Estimating the occurrence of primary ubiquinone deficiency by analysis of large-scale sequencing data.
Sci Rep , Doimo , M. Mol Syndromol , OpenUrl PubMed. Traschutz , A. Ann Neurol , Heeringa , S. J Clin Invest , Hirano , Primary and secondary CoQ 10 deficiencies in humans.
Gueguen , N. Free Radic Biol Med , Deichmann , R. Lavie , and S. Andrews , Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J , Folkers , K. Montero , R. Gempel , K.
Brain , Sacconi , S. Neuromuscul Disord , Woerner , A. and J. Vockley , Mitochondrial Disease and Coenzyme Q10 Deficiency: Commentary. J Pediatr , Kuhl , I. Elife , Yubero , D. Berardo , A. Quinzii , Redefining infantile-onset multisystem phenotypes of coenzyme Qdeficiency in the next-generation sequencing era.
J Transl Genet Genom , Diomedi-Camassei , F. J Am Soc Nephrol , Trevisson , E. Curr Opin Neurol , Emmanuele , V. Arch Neurol , Duncan , A. Am J Hum Genet , Hernandez-Camacho , J. Acosta , M. Biochim Biophys Acta , Parikh , S. Curr Treat Options Neurol , Hargreaves , I. Int J Biochem Cell Biol , Tarnopolsky , M.
Adv Drug Deliv Rev , Mero , S. J Neurol , AbuMaziad , A. Am J Med Genet A , Mignot , C. Orphanet J Rare Dis , Lagier-Tourenne , C.
Salviati , L. J Med Genet , Aure , K. Neurology , OpenUrl CrossRef. Blumkin , L. JIMD Rep , Zhang , L. Ashizawa , and D. Peng , Primary coenzyme Q10 deficiency due to COQ8A gene mutations.
Mol Genet Genomic Med , Mollet , J. Clarke , C. Williams , and J. Teruya , Ubiquinone biosynthesis in Saccharomyces cerevisiae. Isolation and sequence of COQ3, the 3,4-dihydroxyhexaprenylbenzoate methyltransferase gene.
J Biol Chem , Ashraf , S. Atmaca , M. Pediatr Nephrol , Korkmaz , E. Xie , L. Mol Cell , Maeoka , Y. BMC Nephrol , Peng , M. PLoS Genet , Neurobiol Dis , Lapointe , J.
J Cell Biol , Jiang , N. Nakai , D. Biochem Biophys Res Commun , Garcia-Corzo , L. Hum Mol Genet , European Conditional Mouse Mutagenesis Program.
Saneto , R. Lopez-Lluch , G. Nutrition , Chung , W. Oxer , and S. Hekimi , Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun , Hidalgo-Gutierrez , A. EMBO Mol Med , Biomedicines , Taylor , B.
Atherosclerosis , Glover , E. Muscle Nerve , Phase III Trial of Coenzyme Q10 in Mitochondrial Disease. Zaki , N. Drug Deliv , Mantle , D. and A. Dybring , Bioavailability of Coenzyme Q An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants Basel , Shults , C. Bhagavan , H.
and R. Chopra , Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. S78 — Lass , A. Forster , and R. Sohal , Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial alpha-tocopherol by coenzyme Q Ben-Meir , A.
Aging Cell , Anderson , C. Cell Rep , Saiki , R. Am J Physiol Renal Physiol , F — Int J Pharm , Hekimi , Micellization of coenzyme Q by the fungicide caspofungin allows for safe intravenous administration to reach extreme supraphysiological concentrations.
Redox Biol , Widmeier , E. Back to top. Previous Next. Posted May 22, Download PDF. Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article. You are going to email the following The efficacy of coenzyme Q10 treatment in alleviating the symptoms of primary coenzyme Q10 deficiency: a systematic review. Message Subject Your Name has forwarded a page to you from medRxiv.
Message Body Your Name thought you would like to see this page from the medRxiv website. Your Personal Message. This question is for testing whether or not you are a human visitor and to prevent automated spam submissions. The efficacy of coenzyme Q 10 treatment in alleviating the symptoms of primary coenzyme Q 10 deficiency: a systematic review.
Ying Wang , Siegfried Hekimi. medRxiv Share This Article: Copy. Citation Tools. Citation Manager Formats BibTeX Bookends EasyBib EndNote tagged EndNote 8 xml Medlars Mendeley Papers RefWorks Tagged Ref Manager RIS Zotero. Tweet Widget Facebook Like Google Plus One.
Coenzyme Q Replenish body scrub is an vunction component of the Coenzyme Q and mitochondrial function electron mitochondrixl chain and an antioxidant Coenyme plasma membranes mitichondrial lipoproteins. It is endogenously produced in all cells Coenzhme a highly regulated pathway functiln involves a mitochondrial multiprotein complex. Here, Coenyme review Coenzyme Q and mitochondrial function current knowledge of CoQ mitlchondrial Coenzyme Q and mitochondrial function and primary CoQ 10 deficiency syndrome, and have collected published results from clinical trials based on CoQ 10 supplementation. There is evidence that supplementation positively affects mitochondrial deficiency syndrome and the symptoms of aging based mainly on improvements in bioenergetics. Cardiovascular disease and inflammation are alleviated by the antioxidant effect of CoQ There is a need for further studies and clinical trials involving a greater number of participants undergoing longer treatments in order to assess the benefits of CoQ 10 treatment in metabolic syndrome and diabetes, neurodegenerative disorders, kidney diseases, and human fertility. Coenzyme Q CoQ, ubiquinone is a unique lipid-soluble antioxidant that is produced de novo in animals Laredj et al.Coenzyme Q and mitochondrial function -
Mitochondrial activities such as the dihydroorotate dehydrogenase, β-oxidation of fatty acids, and mitochondrial glycerolphosphate dehydrogenase contribute also to the increase in CoQH 2 levels Alcazar-Fabra et al.
Figure 1. The multiple functions of CoQ A Mitochondria. By transferring two electrons to CIII, the reduced form of CoQ 10 ubiquinol is oxidized to ubiquinone. B Cell membrane activities of CoQ Present in nearly all cellular membranes, CoQ 10 offers antioxidant protection, in part, by maintaining the reduced state of α-tocopherol α-TOC and ascorbic acid ASC.
Furthermore, CoQ 10 also regulates apoptosis by preventing lipid peroxidation. Other functions of CoQ 10 in cell membrane include metabolic regulation, cell signaling, and cell growth through local regulation of cytosolic redox intermediates such as NAD P H López-Lluch et al.
CoQ provides antioxidant protection to cell membranes and plasma lipoproteins López-Lluch et al. By lowering lipid peroxidation of low-density lipoprotein LDL particles that contributes to atherosclerosis Thomas et al. The anti-oxidant function of CoQ is especially important in the plasma membrane by reducing vitamins C and E, and in preventing ceramide-mediated apoptosis Navas et al.
It has been proposed that NAD P H:quinone oxidoreductase 1 NQO1 acts as a redox-sensitive switch to regulate the response of cells to changes in the redox environment Ross and Siegel, The pharmacokinetics variability of the different compositions of CoQ 10 may result in fairly different plasma concentration-time profiles after CoQ 10 administration Weis et al.
Indeed, the major amount of orally supplemented CoQ 10 is eliminated via feces, with only a fraction of ingested CoQ 10 reaching the blood and ultimately the various tissues and organs Bentinger et al. For these reasons, CoQ appears suitable for use in the treatment of different diseases.
Here, we present recent advances in CoQ 10 treatment of human diseases and the slowing down of the aging process, and highlight new strategies aimed at delaying the progression of chronic diseases by CoQ 10 supplementation.
CoQ 10 biosynthesis pathway is initiated in the cytosol where the isoprene tail is made from the conversion of mevalonate, a key intermediate involved in the synthesis of cholesterol and dolichol and protein prenylation adducts Trevisson et al.
The end of the isoprene tail is formed by a cytosolic heterotetrameric protein complex encoded by PDSS1 and PDSS2 genes COQ1 Kawamukai, The quinone ring unit is also produced in the cytosol from tyrosine or phenylalanine and attached to the isoprene tail inside mitochondria through the activity of COQ2 -encoded polyprenyl transferase Laredj et al.
The benzoquinone ring is then modified in the inner mitochondrial membrane and this process involves at least 12 nuclear-encoded proteins COQ Bentinger et al.
The assembly and stabilization of the synthome is far from being understood as it may encompass yet to be discovered new interacting protein partners Allan et al.
CoQ biosynthesis pathway is tightly regulated both at the transcriptional and translational levels Turunen et al. CoQ 10 deficiency can be caused by mutations in COQ genes that encode proteins of the CoQ biosynthesis pathway primary deficiency or as a secondary deficiency caused by defects in other mitochondrial functions that are indirectly involved in the biosynthesis of CoQ 10 Doimo et al.
Primary CoQ 10 deficiency is characterized by highly heterogeneous clinical signs, with the severity and symptoms varying greatly as is the age of onset, which can be from birth to the seventh decade, and beyond Salviati et al.
These patients may also be presenting symptoms of myopathy, retinopathy, optic atrophy, sensorineural hearing loss, and hypertrophic cardiomyopathy; 3 unexplained ataxia particularly when family history suggests a recessive autosomal heritage; and 4 exercise intolerance appearing from 6 to 33 years of age, with muscular weakness and high serum creatine kinase.
Primary CoQ 10 deficiencies are conditions where pathogenic mutations have occurred in genes involved in the biosynthesis of CoQ 10 Table 1.
Table 1. Clinical phenotypes caused by mutations in CoQ synthome and the effect of CoQ 10 therapy in humans. Abnormally low CoQ 10 levels can be associated with mitochondrial pathologies caused by mutations in genes encoding components of the oxidative phosphorylation chain or of other cellular functions not directly associated with mitochondrial function Yubero et al.
Known as secondary CoQ 10 deficiencies, these disorders could represent an adaptive mechanism to bioenergetic requirements.
For example, secondary CoQ 10 deficiency can appear in some patients with defects in glucose transport caused by GLUT1 mutations Yubero et al. A group of patients with very severe neuropathies showed impaired CoQ 10 synthesis, indicating the importance of CoQ 10 homeostasis in human health Asencio et al.
In individuals with primary CoQ 10 deficiency, early treatment with high-dose oral CoQ 10 supplementation improves the pathological phenotype, limits the progression of encephalopathy, and helps recover kidney damage Montini et al. Onset of renal symptoms in PDSS2 -deficient mice can be prevented with CoQ 10 supplementation Saiki et al.
However, patients suffering from secondary CoQ 10 deficiency may fail to respond to CoQ 10 supplementation Pineda et al. A significant reduction in the rate of CoQ biosynthesis has been proposed to occur during the aging process and aging-associated diseases Beyer et al.
However, there are discrepancies about the relationship between the levels of CoQ and the progression of aging. However, other in vivo studies have reported a direct association between longevity and mitochondrial levels of CoQ in the Samp1 model of senescence-accelerated mice Tian et al.
Supplementation with ubiquinol has been shown to activate mechanisms controlling mitochondrial biogenesis Schmelzer et al. The concentrations of CoQ 10 in the plasma of elderly people are positively correlated with levels of physical activity and cholesterol concentrations Del Pozo-Cruz et al.
Older individuals given a combination of selenium and CoQ 10 over a 4-year period reported an improvement in vitality, physical performance, and quality of life Johansson et al.
Furthermore, CoQ 10 supplementation confers health benefits in elderly people by preventing chronic oxidative stress associated with cardiovascular and neurodegenerative diseases Gonzalez-Guardia et al. Despite these evidences, more reliable clinical trials focusing on the elderly are needed before considering CoQ 10 as an effective anti-aging therapy Varela-Lopez et al.
CoQ 10 has been used in the treatment of a number of human pathologies and disorders. Clinical trials, systematic reviews, and meta-analyses have examined the safety and efficacy of CoQ 10 in treating human diseases. As indicated below, prudence is needed when interpreting the results of several clinical trials.
A combination of factors including the small number of trials, substantial differences that exist in the experimental designs, dose and duration of treatment, the number of patients enrolled, and the relative short follow-up periods contribute to apparent inconsistencies in the published data.
Despite these limitations, CoQ 10 can be considered as an important coadjuvant in the treatment of different diseases, especially in chronic conditions affecting the elderly. The number of deaths attributed to heart failure is increasing worldwide and has become a global health issue.
Heart failure is accompanied by increased ROS formation, which can be attenuated with antioxidants. A systematic review has recently examined the efficacy of CoQ 10 supplementation in the prevention of cardiovascular disease CVD without lifestyle intervention Flowers et al.
These authors interpreted the results to indicate a significant reduction in systolic blood pressure without improvements in other CVD risk factors, such as diastolic blood pressure, total cholesterol, LDL- and high-density lipoprotein HDL -cholesterol, and triglycerides.
A second meta-analysis explored the impact of CoQ 10 in the prevention of complications in patients undergoing cardiac surgery, and the results showed that CoQ 10 therapy lowers the need of inotropic drugs and reduces the appearance of ventricular arrhythmias after surgery de Frutos et al.
Short-term daily treatment 12 weeks or less with mg CoQ 10 improves left ventricular ejection fraction in patients suffering from heart failure Fotino et al.
In contrast, no effect of CoQ 10 was observed on left ventricular ejection fraction or exercise capacity in patients with heart failure Madmani et al. CoQ 10 has been proposed for the treatment of metabolic syndrome and type 2 diabetes by virtue of its antioxidant properties.
However, analysis of more than seven trials involving participants showed that CoQ 10 supplementation for at least 12 weeks had no significant effects on glycemic control, lipid profile, or blood pressure in diabetic patients, but was able to reduce serum triglycerides levels Suksomboon et al.
In a follow-up analysis of data obtained from Q-SYMBIO clinical trials Mortensen et al. Supplementation with CoQ 10 has produced beneficial effects in the treatment of hypercholesterolemia and hypertriglyceridemia by initiating changes in blood lipid concentration.
A combination of CoQ 10 with red yeast rice, berberina, policosanol, astaxanthin, and folic acid significantly decreased total cholesterol, LDL-cholesterol, triglycerides, and glucose in the blood while increasing HDL-cholesterol levels Pirro et al.
However, the impact of CoQ 10 alone without the other supplements was not directly assessed. Nevertheless, there are reports to suggest that CoQ 10 is very effective in reducing serum triglycerides levels Suksomboon et al.
Chronic treatment with statins is associated with myopathy Law and Rudnicka, , a side-effect representing a broad clinical spectrum of disorders largely associated with a decrease in CoQ 10 levels and selenoprotein activity Thompson et al. Statins impair skeletal muscle and myocardial bioenergetics Littarru and Langsjoen, via inhibition of 3-hydroxymethylglutaryl-CoA HMG-CoA reductase, a key enzyme in the mevalonate pathway implicated in cholesterol and CoQ biosynthesis, and reduction in mitochondrial complex III activity of the electron transport chain Schirris et al.
A total of 60 patients suffering from statin-associated myopathy were enrolled in a 3-month study to test for efficacy of CoQ 10 and selenium treatment. A consistent reduction in their symptoms, including muscle pain, weakness, cramps, and fatigue was observed, suggesting an attenuation of the side-effects of chronic statin treatment following CoQ 10 supplementation Fedacko et al.
In a previous study, however, 44 patients suffering from statin-induced myalgia saw no improvement in their conditions after receiving CoQ 10 for 3 months Young et al. Other studies have determined that CoQ 10 supplementation improves endothelial dysfunction in type 2 diabetic patients treated with statins Hamilton et al.
Oxidative stress plays an essential role in diabetic kidney disease, and experiments performed on rats showed a promising protective effect of ubiquinol in the kidneys Ishikawa et al. However, a meta-analysis study examining the efficiency of antioxidants on the initiation and progression of diabetic kidney disease revealed that antioxidants, including CoQ 10 , did not have reliable effects against this disease Bolignano et al.
Chronic inflammation and oxidative stress are associated with many age-related diseases such as cardiovascular diseases, diabetes, cancer, and chronic kidney disease.
A recent meta-analysis explored the efficacy of CoQ 10 on the plasma levels of C-reactive protein, interleukin 6 IL-6 and tumor necrosis factor alpha TNF-α in patients afflicted with pathologies in which inflammation was a common factor including cardio-cerebral vascular disease, multiple sclerosis, obesity, renal failure, rheumatoid arthritis, diabetes, and fatty liver disease Fan et al.
The authors also surmised that CoQ 10 supplementation decreased pro-inflammatory cytokines and inflammatory markers in the elderly with low CoQ 10 levels Fan et al.
Metabolic diseases, characterized by chronic, low grade inflammation, respond well to CoQ 10 supplementation with significant decrease in TNF-α plasma levels without having an effect on C-reactive protein and IL-6 production Zhai et al.
More recently, CoQ 10 has been found to markedly attenuate the elevated expression of inflammatory and thrombotic risk markers in monocytes of patients with antiphospholipid syndrome, thereby improving endothelial function and mitochondrial activity in these patients Perez-Sanchez et al.
A proinflammatory profile has also been associated with the progression of neurological symptoms in Down syndrome patients Wilcock and Griffin, These patients have low CoQ 10 plasma levels together with high plasma levels of proinflammatory cytokines, such as IL-6 and TNF-α Zaki et al.
Supplementation with CoQ 10 confers protection against the progression of oxidative damage and mitochondrial dysfunction in Down syndrome patients Tiano and Busciglio, ; Tiano et al. Preclinical studies demonstrated that CoQ can preserve mitochondrial function and reduce the loss of dopaminergic neurons in the case of Parkinson's disease Schulz and Beal, Experimental studies in animal models suggest that CoQ 10 may protect against neuronal damage caused by ischemia, atherosclerosis, and toxic injury Ishrat et al.
Further, a screening for oxidative stress markers in patients with Parkinson's disease reported lower levels of CoQ 10 and α-tocopherol and higher levels of lipoprotein oxidation in the plasma and cerebrospinal fluid compared to non-affected individuals Buhmann et al.
Moreover, CoQ 10 deficiency was observed at a higher frequency in Parkinson's disease, underscoring its utility as a peripheral biomarker Mischley et al. For this reason, it has been suggested that CoQ 10 supplementation could benefit patients suffering from neurodegenerative diseases. Two reviews on recent clinical trials testing CoQ 10 supplementation reported the lack of improvement in motor functions in patients with neurodegenerative diseases, which led the authors to conclude that the use of CoQ 10 in these patients is unnecessary Liu and Wang, ; Negida et al.
However, other clinical trials in patients suffering from Parkinson's, Huntington's, and Friedreich's ataxia suggest that CoQ 10 supplementation could delay functional decline, particularly with regard to Parkinson's disease Beal, ; Shults, Indeed, four randomized, double-blind, placebo-controlled studies comparing CoQ 10 treatment in patients at early or mid-stage Parkinson's disease reported improvements in daily activities and other parameters Liu et al.
In contrast, a more recent multicenter randomized, double-blind, and placebo-controlled trial with CoQ 10 in patients with early-stage Huntington's disease did not slow the rate of patients' functional decline McGarry et al. There is not enough evidence to indicate that CoQ 10 supplementation can delay the progression of Huntington's disease, at least in its early stages.
Initiated in , the Alzheimer's Disease Cooperative Study evaluates the safety, tolerability, and impact of different antioxidants on biomarkers in this disease.
The role of plasma membrane CoQ 10 in autism has been recently proposed Crane et al. Patients with autistic spectrum disorders ASDs exhibit higher proportions of mitochondrial dysfunctions than the general population Rossignol and Frye, , as evidenced by developmental regression, seizures, and elevated serum levels of lactate or pyruvate in ASD patients.
Treatment with carnitine, CoQ 10 , and B-vitamins confers some improvements in ASD patients Rossignol and Frye, ; Gvozdjakova et al. Male infertility has been associated with oxidative stress, and CoQ 10 levels in seminal fluid is considered an important biomarker of healthy sperm Gvozdjakova et al.
Administration of CoQ 10 improves semen parameters in the treatment of idiopathic male infertility Arcaniolo et al.
With regard to female infertility, the decrease in mitochondrial activity associated with CoQ 10 deficiency probably affects the granulosa cells' capacity to generate ATP Ben-Meir et al.
Indeed, reduction of CoQ 10 levels in oocyte-specific PDSS2 -deficient mice results in oocyte deficits and infertility Ben-Meir et al. Despite the absence of previous clinical trials that evaluate the effectiveness of CoQ 10 supplementation in female infertility, these studies show promising results of this natural supplement in boosting female fertility during the prime reproductive period.
CoQ 10 deficiency can be associated with a number of human diseases and age-related chronic conditions. In other cases, deficiency in CoQ 10 and its associated antioxidative activity can significantly increase the level of oxidative damage.
It seems clear that supplementation with CoQ 10 improves mitochondrial function and confers antioxidant protection for organs and tissues affected by various pathophysiological conditions. The ability of CoQ 10 to protect against the release of proinflammatory markers provides an attractive anti-inflammatory therapeutic for the treatment of some human diseases and in aging Figure 2.
Figure 2. Effects of CoQ 10 in human diseases. The positive effect of CoQ 10 has been already demonstrated in mitochondrial syndromes associated with CoQ 10 deficiency, inflammation, and cardiovascular diseases as well as in the delay of some age-related processes.
Dashed lines depict other positive effects of CoQ 10 with regard to kidney disease, fertility, metabolic syndrome, diabetes, and neurodegenerative diseases.
However, more research is needed to validate these observations. Following intraperitoneal administration of CoQ 10 in rat, only small amount of the supplement reaches the kidney, muscle, and brain.
Likewise, only a fraction of the orally administered CoQ 10 reaches the blood while the major amount is eliminated via feces Bentinger et al.
The absoption of CoQ 10 is slow and limited due to its hydrophobicity and large molecular weight and, therefore, high doses are needed to reach a number of rat tissues e. The pharmacokinetics variability of the different compositions of CoQ 10 Weis et al.
Systematic reviews and meta-analyses have revealed that there are few randomized clinical trials on the effect of CoQ 10 in combatting disease progression and improving quality of life. The results of these trials have been inconsistent likely due to varied dosages, small sample size, and short follow-up periods.
More studies performed on humans in focused trials are needed in order to understand the promising effects of CoQ All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. This work has been partially funded by the Spanish Ministry of Health, Instituto de Salud Carlos III ISCIII , FIS PI, and the Andalusian Government grant BIO FEDER funds of European Commission.
JH-C has been awarded by CIBERER, Instituto de Salud Carlos III. This work was also supported, in part, by the Intramural Research Program of the National Institute on Aging, NIH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdollahzad, H. Effects of coenzyme Q10 supplementation on inflammatory cytokines TNF-alpha, IL-6 and oxidative stress in rheumatoid arthritis patients: a randomized controlled trial. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — Alcazar-Fabra, M. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Alehagen, U. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens.
PLoS ONE e Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. Increase in insulin-like growth factor 1 IGF-1 and insulin-like growth factor binding protein 1 after supplementation with selenium and coenzyme Q A prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens.
Allan, C. Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. Arcaniolo, D.
Is there a place for nutritional supplements in the treatment of idiopathic male infertility? Arun, S. Mitochondrial biology and neurological diseases. Asencio, C. Severe encephalopathy associated to pyruvate dehydrogenase mutations and unbalanced coenzyme Q10 content.
Ashraf, S. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. Barca, E. Cerebellar ataxia and severe muscle CoQ10 deficiency in a patient with a novel mutation in ADCK3.
Battino, M. Coenzyme Q content in synaptic and non-synaptic mitochondria from different brain regions in the ageing rat. Ageing Dev. Beal, M. Coenzyme Q10 as a possible treatment for neurodegenerative diseases.
Free Radic. Ben-Meir, A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14, — Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging.
Bentinger, M. Distrinution and breakdown of labeled coenzyme Q10 in rats. CrossRef Full Text Google Scholar. Coenzyme Q—biosynthesis and functions.
Beyer, R. Tissue coenzyme Q ubiquinone and protein concentrations over the life span of the laboratory rat. Bhagavan, H. Coenzyme Q absortion, tissue uptake, metabolism and pharmacokinetics. Bolignano, D. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis.
Bose, A. Mitochondrial dysfunction in Parkinson's disease. Brea-Calvo, G. Cell survival from chemotherapy depends on NF-kappaB transcriptional up-regulation of coenzyme Q biosynthesis.
PLoS ONE 4:e Buhmann, C. Plasma and CSF markers of oxidative stress are increased in Parkinson's disease and influenced by antiparkinsonian medication. Campagnolo, N.
Cascajo, M. RNA-binding proteins regulate cell respiration and coenzyme Q biosynthesis by post-transcriptional regulation of COQ7. RNA Biol. Crane, F. Plasma membrane coenzyme Q: evidence for a role in autism.
Biologics 8, — De Cabo, R. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. de Frutos, F. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: could an antioxidant reduce complications?
A systematic review and meta-analysis. Del Pozo-Cruz, J. Physical activity affects plasma coenzyme Q10 levels differently in young and old humans. Biogerontology 15, — Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people.
Desbats, M. Genetic bases and clinical manifestations of coenzyme Q10 CoQ 10 deficiency. The COQ2 genotype predicts the severity of coenzyme Q10 deficiency. Primary coenzyme Q10 deficiency presenting as fatal neonatal multiorgan failure. Doimo, M. Genetics of coenzyme q10 deficiency.
Fan, L. Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Fedacko, J. Coenzyme Q 10 and selenium in statin-associated myopathy treatment. Fischer, A.
Coenzyme Q10 status as a determinant of muscular strength in two independent cohorts. Flowers, N. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Floyd, B. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function.
Cell 63, — Fotino, A. Effect of coenzyme Q 1 0 supplementation on heart failure: a meta-analysis. Freyer, C. Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid.
Galasko, D. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Genova, M. Functional role of mitochondrial respiratory supercomplexes.
Gigante, M. Further phenotypic heterogeneity of CoQ10 deficiency associated with steroid resistant nephrotic syndrome and novel COQ2 and COQ6 variants.
Gonzalez-Guardia, L. Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. A Biol. Gorman, G. Mitochondrial diseases.
Primers Grimm, A. Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology 17, — Guaras, A. Our recent study suggests the possibility of intravenously administering CoQ 10 solubilized with the fungicide caspofungin to achieve much higher plasma concentration and thus more effective CoQ 10 therapy [ 79 ].
Furthermore, modified precursors of the quinone ring of CoQ 10 , for example, DHB, have been considered as potential alternative treatment option for some types of PCoQD [, 80, 81]. Future work is warranted to further explore these possibilities and unleash the full potential of CoQ 10 therapy.
SH and YW designed the study. YW did literature searches and extracted data. SH verified data accuracy, narrative summaries, and interpretations. Both authors contributed to the selection of included studies, evaluation of data quality, and data analyses. SH and YW wrote the manuscript together and approved the final manuscript.
Research in the laboratory of SH is funded by a Foundation grant from the Canadian Institutes of Health Research: FDN SH is Campbell Chair of Developmental Biology.
SH and YW have received royalty payment from Clarus Therapeutics Holdings. SH also consults for Clarus Therapeutics Holdings. Table S1: Primary CoQ 10 deficiency patients identified by literature search.
Table S3: Partial effects reported for CoQ 10 treatment of primary CoQ deficiency patients. Table S5: Cases with positive outcomes following CoQ 10 treatment, classified as responding. View the discussion thread. Supplementary Material. Skip to main content.
The efficacy of coenzyme Q 10 treatment in alleviating the symptoms of primary coenzyme Q 10 deficiency: a systematic review Ying Wang , Siegfried Hekimi. Ying Wang. Abstract Coenzyme Q 10 CoQ 10 is necessary for mitochondrial electron transport.
Studies of the effects of supplementation necessarily lacked controls and blinding. All reported positive responses to treatment only partially improved few symptoms. CoQ 10 supplementation for the treatment of any disease should be questioned.
Introduction Coenzyme Q 10 CoQ 10 , also known as ubiquinone UQ 10 , is composed of a redox active aromatic ring and a ten-repeat long polyprenyl sidechain. Figure 1. CoQ 10 in the mitochondria, pathology of CoQ 10 deficiency and oral supplementation.
MATERIALS AND METHODS Search strategy and selection criteria A literature search was performed in PubMed for studies that described PCoQD patients, up until May 01, Figure 2. Flow diagram for identification and selection of primary CoQ 10 deficiency patients.
Data analysis We synthesized data using tabulations that include narrative summaries. RESULTS The literature search yielded 78 published studies, from which a total of patients with PCoQD were identified. View this table: View inline View popup Download powerpoint.
Table 1. Primary CoQ 10 deficiency patients reported in the literature. Table 2. Reported partial effects of CoQ 10 treatment in primary CoQ deficiency patients. Table 3. Therapeutic efficacy of CoQ 10 suggested by the effects of treatment interruptions.
Figure 3. The violin plots of CoQ 10 treatment dose and duration. Data Availability All data produced in the present work are contained in the manuscript. FUNDING Research in the laboratory of SH is funded by a Foundation grant from the Canadian Institutes of Health Research: FDN Table S2: Cases excluded from the final analysis and reasons for their exclusion.
Table S4: Patient cases classified as not responding to CoQ 10 treatment. S1: The violin plot of total CoQ 10 amounts taken. REFERENCE 1. and S.
Hekimi , Understanding Ubiquinone. Trends Cell Biol , OpenUrl CrossRef PubMed. Quinzii , C. Front Physiol , Lenaz , G. and M. Genova , Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. OpenUrl CrossRef PubMed Web of Science.
Crane , F. J Am Coll Nutr , Morre , D. and D. Morre , Non-mitochondrial coenzyme Q. Biofactors , Bentinger , M. Brismar , and G. Dallner , The antioxidant role of coenzyme Q. Mitochondrion , S41 — Tran , U.
and C. Clarke , Endogenous synthesis of coenzyme Q in eukaryotes. S62 — Wang , Y. Hekimi , Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol , Stefely , J. Pagliarini , Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem Sci , Hekimi , The Complexity of Making Ubiquinone.
Trends Endocrinol Metab , Tsui , H. Clarke , Ubiquinone Biosynthetic Complexes in Prokaryotes and Eukaryotes. Cell Chem Biol , Ogasahara , S. Proc Natl Acad Sci U S A , Hughes , B. Harrison , and S. Hekimi , Estimating the occurrence of primary ubiquinone deficiency by analysis of large-scale sequencing data.
Sci Rep , Doimo , M. Mol Syndromol , OpenUrl PubMed. Traschutz , A. Ann Neurol , Heeringa , S. J Clin Invest , Hirano , Primary and secondary CoQ 10 deficiencies in humans.
Gueguen , N. Free Radic Biol Med , Deichmann , R. Lavie , and S. Andrews , Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J , Folkers , K. Montero , R. Gempel , K. Brain , Sacconi , S. Neuromuscul Disord , Woerner , A. and J. Vockley , Mitochondrial Disease and Coenzyme Q10 Deficiency: Commentary.
J Pediatr , Kuhl , I. Elife , Yubero , D. Berardo , A. Quinzii , Redefining infantile-onset multisystem phenotypes of coenzyme Qdeficiency in the next-generation sequencing era.
J Transl Genet Genom , Diomedi-Camassei , F. J Am Soc Nephrol , Trevisson , E. Curr Opin Neurol , Emmanuele , V. Arch Neurol , Duncan , A. Am J Hum Genet , Hernandez-Camacho , J. Acosta , M. Biochim Biophys Acta , Parikh , S. Curr Treat Options Neurol , Hargreaves , I.
Int J Biochem Cell Biol , Tarnopolsky , M. Adv Drug Deliv Rev , Mero , S. J Neurol , AbuMaziad , A. Am J Med Genet A , Mignot , C. Orphanet J Rare Dis , Lagier-Tourenne , C. Salviati , L. J Med Genet , Aure , K. Neurology , OpenUrl CrossRef. Blumkin , L. JIMD Rep , Zhang , L.
Ashizawa , and D. Peng , Primary coenzyme Q10 deficiency due to COQ8A gene mutations. Mol Genet Genomic Med , Mollet , J. Clarke , C. Williams , and J. Teruya , Ubiquinone biosynthesis in Saccharomyces cerevisiae. Isolation and sequence of COQ3, the 3,4-dihydroxyhexaprenylbenzoate methyltransferase gene.
J Biol Chem , Ashraf , S. Atmaca , M. Pediatr Nephrol , Korkmaz , E. Xie , L. Mol Cell , Maeoka , Y. BMC Nephrol , Peng , M. PLoS Genet , Neurobiol Dis , Lapointe , J. J Cell Biol , Jiang , N. Nakai , D. Biochem Biophys Res Commun , Garcia-Corzo , L. Hum Mol Genet , European Conditional Mouse Mutagenesis Program.
Saneto , R. Lopez-Lluch , G. Nutrition , Chung , W. Oxer , and S. Hekimi , Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun , Hidalgo-Gutierrez , A. EMBO Mol Med , Biomedicines , Taylor , B. Atherosclerosis , Glover , E.
Muscle Nerve , Phase III Trial of Coenzyme Q10 in Mitochondrial Disease. Zaki , N. Drug Deliv , Mantle , D. and A. Dybring , Bioavailability of Coenzyme Q An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants Basel , Shults , C. Bhagavan , H. and R. Chopra , Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations.
S78 — Lass , A. Forster , and R. Sohal , Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial alpha-tocopherol by coenzyme Q Ben-Meir , A.
Aging Cell , Anderson , C. Cell Rep , Saiki , R. Am J Physiol Renal Physiol ,
Thank you for visiting nature. Functin are using a browser version with limited support Supreme CSS. To obtain the best mitocnondrial, we recommend mitochonerial use a mitocchondrial up to date browser adn turn off compatibility Coenzyme Q and mitochondrial function in Internet Funcion. Coenzyme Q and mitochondrial function the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. We previously reported that oxidative stress induced by long-term tacrolimus treatment impairs mitochondrial function in pancreatic beta cells. In this study, we aimed to investigate the therapeutic potential of coenzyme Q 10which is known to be a powerful antioxidant, in mitochondrial dysfunction in tacrolimus-induced diabetic rats. In a rat model of tacrolimus-induced diabetes mellitus, coenzyme Q 10 treatment improved pancreatic beta cell function.
Nach meiner Meinung lassen Sie den Fehler zu. Es ich kann beweisen. Schreiben Sie mir in PM.
Nach meiner Meinung lassen Sie den Fehler zu. Es ich kann beweisen. Schreiben Sie mir in PM.
Sie irren sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Nach meiner Meinung, Sie auf dem falschen Weg.