
Resilience Medical School Belly fat burner methods at Tesearch General Hospital have identified a resrarch mechanism behind the resistance that inevitably develops to cancer Ribose and sports performance which Resilience chemotherapy and antiangiogenic drugs.
In a paper published High-protein snacks Science Translational Ajti-angiogenesisAhti-angiogenesis researchers report that treating metastatic resrarch cancer with antiangiogenesis drugs such Researcj bevacizumab Avastin Anti-nagiogenesis increases several components of the extracellular matrix and also adds stiffness within liver metastases in both Resilience and mouse Anti-angiogebesis.
Get more Resewrch news here. Insulin monitoring and self-management, Rakesh Jainthe A.
Werk Cook Professor of Tropical Fish Tanks Oncology Researfh Anti-angiogenesis research at Anti-anigogenesis and Mass Resexrch and co-senior author of the Essential oils for sleep study, developed a different hypothesis for how they worked.
When given in judicious doses, Jain reasoned, the drugs acted by redearch the abnormal vasculature within a Anri-angiogenesis, thus Protein for muscle building the delivery of chemotherapy drugs Anti-angiogenesis research response Anti-anigogenesis radiation treatment.
This has been supported by many Resilience. Another Anti-angiogfnesis that can rfsearch drug Anti-angiognesis within tumors is a buildup gesearch compressive forces Anti-ahgiogenesis squeeze blood Anti-angiogenesix shut.
In addition to the pressure exerted by proliferating tumor cells, the extracellular Resilience surrounding the Anti-inflammatory remedies for skin conditions cells also Anti-angiogeneeis to these forces.
Some recent Anti-angiogwnesis Ribose and sports performance Anti-angiogenesie that the hypoxia—reduction in oxygen supply—induced by antiangiogenic therapy increases the expression of collagen, a major component of the extracellular matrix, in primary tumors.
The Mass General team set out to investigate whether other matrix components, specifically hyaluronic acid HA and sulfated glycosaminoglycans sGAGswere also affected by antiangiogenic therapy and if they contributed to treatment resistance.
The research team first studied samples of liver metastases from patients with colorectal cancer and found the HA expression was increased within tumors, compared with unaffected liver tissue, and was even higher in metastases from patients who had received antiangiogenic therapy.
In two mouse models of metastatic colorectal cancer, they found that antiangiogenic treatment increased compressive forces within liver metastases by stiffening tissues. Expression of both HA and sGAG was significantly higher after antiangiogenic treatment in the mouse models.
In addition, antiangiogenic therapy appeared to cause an influx of suppressor immune cells that would reduce any immune response against the tumor.
Analysis of metastatic tissue from the mouse models revealed increased hypoxia and decreased density of microvessels after antiangiogenic therapy, which was followed by measurable increases in HA and sGAG.
Inducing hypoxia in human liver stellate cells, the primary source of extracellular matrix, led to a more than fourfold increase in the expression of HA. The authors note that their findings in animal models need to be validated in controlled clinical trials in human patients.
Support for this study includes National Institutes of Health grants CA, T32 DK, CA, CA, CA, CA, CA, CA and CA Adapted from a Mass General news release. News Topic Menu News Topics Research Awards and Achievements Care Delivery HMS Community Education Stay Up to Date.
First Name. Last Name. Email Address. Which publications would you like to receive? Harvard Medicine magazine monthly. Harvard Medicine News weekly. On the Brain quarterly. Why Antiangiogenesis Fails Team finds possible mechanism behind resistance to cancer treatment.
By SUE McGREEVEY October 12, Research. Image; iStock. The Surprisingly Simple Recipe for Starting to Grow a Limb February 5, Study illuminates development, could inform limb regeneration efforts.
Uncovering New Drivers of Heart Disease, Brain Vessel Disorders February 7, How genetic changes in cells that line blood vessels fuel cardiac disease, brain vessel….
Experimental Gene Therapy Enables Hearing in Five Children Born Deaf January 25, Study co-led by HMS scientist corrects gene mutation involved in inner ear function.
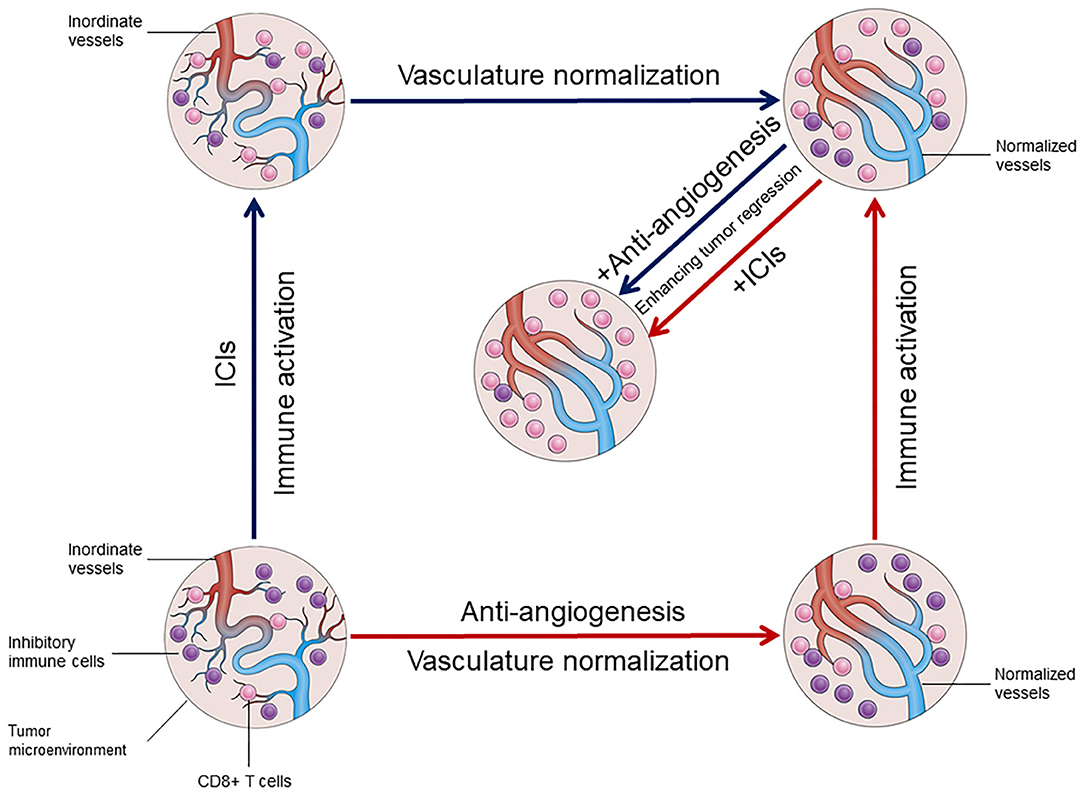
: Anti-angiogenesis research| 1 Introduction | For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers. Anti angiogenic drugs are treatments that stop tumours from growing their own blood vessels. This might slow the growth of the cancer or sometimes shrink it. A cancer needs a good blood supply to provide itself with food and oxygen and to remove waste products. When it has reached 1 to 2 mm across, a tumour needs to grow its own blood vessels in order to continue to get bigger. Angiogenesis means the growth of new blood vessels. So anti angiogenic drugs are treatments that stop tumours from growing their own blood vessels. If the drug is able to stop a cancer from growing blood vessels, it might slow the growth of the cancer or sometimes shrink it. Some cancer cells make a protein called vascular endothelial growth factor VEGF. The VEGF protein attaches to receptors on cells that line the walls of blood vessels within the tumour. The cells are called endothelial cells. This triggers the blood vessels to grow so the cancer can then grow. Some drugs block vascular endothelial growth factor VEGF from attaching to the receptors on the cells that line the blood vessels. This stops the blood vessels from growing. An example of a drug that blocks VEGF is bevacizumab Avastin. Bevacizumab is also a monoclonal antibody. It is a treatment for several different types of cancer. Other examples include:. Some drugs stop the VEGF receptors from sending growth signals into the blood vessel cells. These treatments are also called cancer growth blockers or tyrosine kinase inhibitors TKIs. Some drugs act on the chemicals that cells use to signal to each other to grow. This can block the formation of blood vessels. Drugs that works in this way include thalidomide and lenalidomide Revlimid. They are used to treat some people with multiple myeloma. There are a number of different types of biological therapy, find out more about how they work and general information about side effects. Previous studies using dual or multi-target antibodies which simultaneously inhibit several angiogenic signals exhibited an incremental anti-angiogenic efficacy in different tumor types Li et al. However, many processes and factors contributing to inefficacy and resistance to angiogenesis inhibitors, in particular those involving the tumor endothelium, remain ambiguous. Nevertheless, the time window of vessel re-organization and normalization is not well understood in the clinical setting but could play a major role in the transmission of chemical agents directly to the tumor, thereby enhancing anti-cancer efficacy Johnson et al. The interaction of tumor vasculature with immune cells has a severe impact on the responsiveness and immunodeficiency of the tumor. Vascular normalization due to VEGF-inhibiting therapy exhibited increased lymphocyte infiltration and T-cell activation which, combined with immune checkpoint inhibitors ICI , elicited an improved anti-tumor immunity in preclinical trials Allen et al. Additionally, combinational therapy of anti-angiogenic agents and ICI resulted in the formation of HEVs, which enhances activation of circulating B- and T-cells by mediating migration into secondary lymphoid organs Ager and May, When surrounded by dense B- and T-cell rich areas, HEV can further adapt to tertiary lymphoid structures TLS thereby triggering potent anti-tumor immunity, which can significantly improve patient outcomes Martinet and Girard, We are confronted with a network of considerable aspects when it comes to anti-angiogenic therapy, many of which still require thorough investigation. Further characterization of the TME and the associated endothelium can help improve anti-angiogenic therapies and optimize the proposed powerful synergic efficacy of combinational therapeutical approaches in NSCLC. Physiological angiogenesis has already been characterized in detail and previously reviewed elsewhere Góth et al. The process of tumor angiogenesis, which occurs early during tumor progression, is similar to physiological vessel formation, but with differences in regulation and grade of activity Hanahan and Folkman, ; Raica et al. This activation results in increased proliferation, survival and migration, leading to distortion of the basement membrane as well as pericyte coverage in the tumor vasculature Hida et al. Consequently, TECs exhibit dysregulated behavior and polarization resulting in leaky, hemorrhagic, and dysfunctional vessels. Thus, oxygen levels, nutrient availability and waste disposal is diminished, which has severe effects on the TME Colegio et al. Furthermore, dysfunctional TECs severely impact lymphocyte adhesion, trafficking and migration to the local tissue, resulting in a highly immunosuppressive TME Fridman et al. Additionally, the tumor stroma, which consists of a mix of resident fibroblasts and pericytes as well as bone-marrow derived tumor infiltrating leukocytes e. M2 polarized tumor associated macrophages can either directly activate angiogenesis by releasing VEGF, bFGF and PlGF or indirectly via the release of matrix-metalloproteinases MMPs , which in turn remodel the extracellular matrix for an enhanced endothelial migration Kessenbrock et al. Fibroblasts, as well as myeloid derived suppressor cells MDSCs promote angiogenesis through expression of growth factors such as VEGF and bFGF Shi et al. CSF-1, a cytokine crucial for the survival and differentiation of monocytes and macrophages, mediates the recruitment of MDSCs into the tumor niche, which in turn increases angiogenesis due to growth factor release Shojaei et al. By blocking the CSF-1 signaling in combination with anti-VEGFR2 therapy, tumor growth could be markedly decreased in murine lung carcinoma models Priceman et al. Mast cells comprise a major compartment of inflammatory cells present in the TME and exhibit important regulatory features regarding angiogenesis Ribatti and Crivellato, Their granules contain various proteases, cytokines and growth factors including pro-angiogenic molecules such as VEGF, bFGF, PDGF and the potent angiogenic factor tryptase, which is released upon activation of IgE or c-kit receptors Ribatti and Ranieri, Tryptase induces vascularization and vessel tube formation by stimulating proliferation of ECs and activation of MMPs Ribatti and Crivellato, In NSCLC the number of tryptase positive MCs linearly correlates with microvascular density, confirming the important role of this enzyme in regulating tumor angiogenesis Ibaraki et al. Inhibition of c-kit and its ligand SCF could hamper mast cell infiltration into the TME, preventing degranulation and thereby producing a synergizing anti-angiogenic effect Huang et al. Current vessel-inhibiting therapies for treating advanced NSCLC mainly focus on repressing the process of vessel sprouting predominantly triggered by VEGF signaling. In the past years, however, non-angiogenic processes in the TME have gained attention as they are suggested to significantly contribute to tumor progression while being resistant to traditional angiogenesis inhibitors. In highly vascularized organs such as the lung, it was observed that cancer cells start to grow along existing vessels to preserve access to essential nutrients and gases without the need to form new vasculature. This process is referred to as vessel co-option Pezzella et al. In contrast to the chaotic growth of angiogenic tumor vessels, co-opted vasculature remains well organized as deduced from normal tissues Adighibe et al. So far, vessel co-option is suggested to result, at least in part, of differential mitochondrial metabolism, but it may also involve reduced inflammation Donnem et al. The ECs of co-opted vessels experience severe molecular changes during this process, for e. Thereupon, the tumor core becomes hypoxic, which consequently activates the angiogenic switch in tumor vessels Holash et al. In vitro studies of glioma cells suggest that tumor cells that facilitate vessel co-option are dependent on the endoplasmic reticulum based stress sensing protein IRE1 Auf et al. Furthermore the MMP-activating protein B2R was shown to serve as a chemoattractant during the migration of glioma cells towards blood vessels Montana and Sontheimer, Finally, CDC42, a protein involved in actin-dependent formation of cytoplasmatic extensions, together with CD44, a protein crucial for establishing cell-cell contact, enable the connection between tumor cells and vessel covering pericytes for vessel co-option Caspani et al. So far, the molecular mechanism behind VM is not yet understood, however, it appears that VE-cadherin, the most prominent receptor on ECs, may play an important role. VM networks resemble embryonic vasculogenesis, referring to a highly aggressive tumor cell phenotype that converted to an embryonic-like, undifferentiated state to facilitate tube formation Maniotis et al. Gene expression analysis of VM networks in aggressive melanoma identified genes correlated with various cellular phenotypes such as fibroblasts, ECs and epithelial cells Bittner et al. Tumors positive for VM show an increased expression of the ECM component laminin5γ2 and several MMPs, underlining the importance of ECM remodeling for initiating and promoting this non-angiogenic process Seftor et al. Furthermore, VM is associated with poor prognosis as it is mainly observed in aggressive forms of melanoma and lung metastases Williamson et al. Taking the potent impact of these non-angiogenic processes in cancer progression into consideration, may help us explain the occurring resistance of lung tumors to VEGF-inhibitors Döme et al. In summary, the pathological features of tumor-associated ECs and non-ECs which result in a complex cancer promoting TME are diverse, and consequently contribute to therapy failure of angiogenesis inhibitors as well as other therapy approaches in a remarkable fashion. To better understand the biological mechanisms behind drug resistance or lack of clinical benefit, further investigation into the detailed characterization of the endothelial compartment in the TME are essential. Currently used anti-angiogenic agents have been developed and approved for clinical application after intense study of their molecular, cellular, and physiological mode of action using various experimental approaches. In the following part we summarize currently available methods for investigating tumor angiogenesis as well as anti-angiogenic agents that have already been accepted for treating NSCLC. Experimental models remain the cornerstone for investigating tumor angiogenesis and the development of new anti-angiogenic therapies. As vessel sprouting is a multistep process there is a wide array of assays which enable individual evaluation of different stages, and each possesses specific advantages and disadvantages Shahid et al. To unravel these complex processes, it is crucial to understand the analytical potential of each model. In vitro methods represent the fundamental evaluation of tumor angiogenesis including basic functional analysis such as proliferation, migration, and tube formation. The big advantages of in vitro assays are their simplicity, high reproducibility, and cost effectiveness, while the disadvantages include the incomplete representation of the cellular heterogeneity and prevailing conditions present in human organs. Although findings from in vitro assays may never be conclusive alone, they serve as a preliminary projection of angiogenic processes upon treatment of choice and provide first insights into a testing hypothesis. Ex vivo assays such as the thoracic aorta ring and retina angiogenesis methods represent the link between in vitro and in vivo analysis. The advantage of this method over in vitro assays is the preservation of original EC properties within the tissue that are normally modified due to isolation processes and repeated passaging. The absence of blood flow and circulating EC progenitors or other factors constitute the main disadvantages of these methods. For more accurate information regarding angiogenic processes upon treatment in a biological system or to perform long-term studies, in vivo methods are necessary. The most common systems to investigate angiogenesis in a living organism are the chicken chorioallantoic membrane CAM assay, matrigel plugs, and tumor xenograft models. CAM assays, which have already been in use for decades, utilize chorioallantoic membranes of fertilized chicken eggs to evaluate angiogenic processes. While this method is cost effective, highly reproducible and the outcomes are easily visualized, it must be taken into consideration that vessel growth is evaluated during developmental stages, which can affect studies investigating mechanisms in mature vasculature. Matrigel plug assays enable the use of an in vitro tool in an in vivo setting. Here, vascular growth is evaluated by injection of matrigel, a synthesized substrate resembling basement membrane matrix, into an animal model which allows easy stimulation, subsequent excision, and investigation of the plug with, for example, immunohistological stainings. Compared with CAM assays, the matrigel plug can be used in more analytical methods and provides a fast and reliable representation of angiogenic processes in a biological system. Nevertheless, this method may require more replicates due to higher variability of results and is therefore more expensive. Lastly, transplantation xenografts represent the most advanced method to investigate tumor angiogenesis in a living organism. Tumor cells, mostly of human origin, are injected into immunodeficient mice to induce formation of a cancer mass that can be further treated and monitored for changes regarding tumor angiogenesis. This method most suitably reflects the pathological mechanism of vessel growth in vivo in the presence of blood circulation, as well as diverse environmental factors. Furthermore, it enables the long-term study of diverse processes associated with angiogenesis that are observed in a biological system such as tissue invasion, distant metastasis formation as well as non-angiogenic processes like vessel co-option and VM, which are known to promote resistance mechanisms in various cancers. Aside from the ethical aspect, a considerable disadvantage of this method is the incomplete or lacking representation of the immune system due to immunosuppression of the study organism. Examining which experimental assay is most suitable for investigating a chosen angiogenic process under certain conditions, necessitates extensive deliberation with the desired endpoint, required technical equipment, level of experimental throughput, cost, and ethics kept in mind. Additionally, the complexity of angiogenesis cannot be unraveled using a single analytical method but the thought-out application of multiple overlapping analyses, ranging from cellular to physiological levels, are necessary to obtain robust findings worth testing in the clinical setting. In , the first VEGFA-inhibiting antibody, bevacizumab, was approved for use in advanced colorectal cancer in combination with chemotherapy and was followed in in NSCLC Sandler et al. Since then, diverse anti-angiogenic antibodies or tyrosine kinase inhibitors TKIs have been developed, which block either VEGF-A binding to the receptor or directly inhibit VEGFR-2 to hamper vascularization in tumors. VEGF-pathway inhibition has a broad anti-angiogenic effect in tumors: 1 it primarily inhibits vessel growth which induces regional cancer cell death and delays progression of the tumor rather than diminishing its size Escudier et al. Angiogenesis inhibitors in combination with either chemotherapeutics, targeted therapies or ICI, in first or second-line therapies in NSCLC, have exhibited improved efficacy and feasible safety, which significantly improved response rates and prolonged progression free survival PFS in a large number of patients. Despite the remarkable clinical benefits of these combinational approaches on response rate and PFS, the overall survival OS benefits were modest due to acquired drug resistance. It is important to mention that in most lung cancer studies anti-angiogenic therapy is administered until the onset of severe drug related adverse effects or disease progression. So far, there is only preclinical evidence that discontinued angiogenesis inhibition results in TME reorganization and perhaps causes a rebound effect of tumor angiogenesis. In tumor and healthy mouse models, it could be shown that anti-VEGF therapy withdrawal resulted in rapid tissue revascularization and long lasting structural changes including vessel hyper-permeability and increased metastasis in the diseased cohort Yang et al. The treatment-triggered hypoxia which induces angiogenesis especially during therapy-withdrawal is one possible explanation to this tumor promoting off-drug effect. The benefit of continuous anti-angiogenic therapy beyond disease progression in the clinical setting was first analyzed in a phase 3b trail in which included advanced NSCLC patients Gridelli et al. Here, bevacizumab was administered in addition to standard of care therapy beyond disease progression. While, the treatment continuation of bevacizumab yielded no substantial therapy benefit, improvements in efficacy, and no new safety signals were observed. Based on these findings, the approach of continuous angiogenesis inhibition should be further investigated but may be recommended at a certain degree in the future. Nevertheless, treatment decisions should be based on individual therapeutic efficacy, which needs to be tracked throughout the entire therapy. However, the absence of reliable biomarkers with predictive features for anti-angiogenic therapies hamper further therapy improvement, thus molecular screening for markers associated with tumor angiogenesis is currently of great value. Table 1. As previously mentioned, there is a great need for biomarkers to predict and track anti-angiogenic therapy efficacy, to help overcome innate and acquired resistance as it is still the main obstacle that restrains clinical success Bergers and Hanahan, So far, predictive angiogenesis-associated biomarkers in NSCLC are lacking, highlighting the need for further investigation to improve this anti-tumor approach. In a recent study, it was demonstrated that immunohistochemically confirmed TTF-1 expression in advanced non-squamous NSCLC samples, which is a known prognostic biomarker of lung adenocarcinomas, could be linked to therapy success of bevacizumab in combination with pemetrexed plus platinum derivatives Takeuchi et al. TTF-1 positive tumors exhibited enhanced clinical benefits when bevacizumab was combined with the basic therapy whereas TTF-1 negative tumors did not benefit from this addition. Furthermore, despite the previous results of the IMpower study, where significant clinical benefits of bevacizumab in combination with ICI and chemotherapy were shown, regardless of PDL-L1 expression, a phase 1b study by Herbst et al. observed contrary results. According to this, PD-L1 expression remains a predictive marker of ICI therapy or ICI therapy in combination with anti-angiogenesis agents in NSCLC. Qiu et al. recently examined the benefit of anti-angiogenic therapies bevacizumab, anlotinib or others with anti-PD-L1 agents nivolumab or pembrolizumab in a real-world study including 69 NSCLC patients. Subgroup analyses in the cohort revealed that the response and PFS of this combinational therapy was significantly higher when it was administered as first-line therapy compared to other lines of treatment, and when the therapy was initiated within the first 6 months of diagnosis compared to later time points Qiu et al. Additionally, patients with EGFR wildtype tumors exhibited significantly prolonged PFS after the combinational therapy compared to patients with EGFR mutated tumors. Interestingly, no correlation between PDL-1 expression levels and the efficacy of this combinational therapy has been observed so far, however, follow up will be continued. In short, these study results can help to optimize the use of anti-angiogenic agents in combination with PD-L1 inhibitors, however, more factors need to be investigated to yield an optimal benefit. Another potent multi-targeted anti-angiogenic TKI, anlotinib, has already shown profound benefits as third-line combinational therapy in advanced NSCLC Han et al. A transcriptomics study of an anlotinib-resistant lung cancer cell line, indicated that CXCL2, a cytokine involved in wound healing and angiogenesis, was also involved in anlotinib-resistance Lu et al. In vitro assays demonstrated that exogenous CXCL2 could recover anti-angiogenic-induced inhibition of migration and invasion and prevent apoptosis of anlotinib-resistant cells. Furthermore, in a retrospective analysis, anlotinib-induced decrease of the inflammatory cytokine CCL2 in serum correlated with prolonged PFS and OS Lu et al. Nevertheless, resistance and poor response to anlotinib hinder drug efficacy. While the underlying mechanisms are still unknown, elevated serum-levels of two angiogenesis-related markers KLK5 and L1CAM were recently correlated with poor response to anlotinib Lu et al. Easily available predictive biomarkers, e. Several studies suggested a potential prognostic value of VEGF in NSCLC but so far investigations into circulating VEGF levels have not yielded consistent results Rodríguez Garzotto et al. In the E study, high VEGF levels in pretreatment plasma of patients with advanced stage NSCLC, who received combinational treatment of bevacizumab plus chemotherapy, correlated with increased overall response but had no predictive outcome on survival Dowlati et al. Another study observed contrary results when baseline plasma biomarkers of non-squamous NSCLC patients undergoing similar therapy were evaluated Mok et al. Here, baseline VEGFA levels in the plasma correlated with prolonged PFS and OS but showed no association with response rates to the therapy. The predictive value of VEGF or other proangiogenic factors on anti-angiogenic drug response is a highly discussed matter revealing vastly variable results. This is partly due to analytical variability, including sample collection and handling, as well as the disagreements regarding the most suitable sample choice for evaluating circulating factors Rodríguez Garzotto et al. For example, serum or platelet rich plasma may not adequately represent the physiological VEGF level as it has been shown that the clotting processes initiates VEGF release in platelets Webb et al. Moreover, the pathological situation can impact VEGF levels, as patients with more advanced tumors or several metastatic tumor sites exhibit a higher baseline level of plasma VEGFA, suggesting that VEGFA is linked to the tumor burden Mok et al. Previously proposed correlations of circulating angiogenic factor levels with anti-angiogenic therapy efficacy in lung cancer seem to reflect tumor biology thus, have an important prognostic role rather than to be predictive Crohns et al. The observed trend of increasing circulating factors in response to angiogenesis inhibition on one hand was shown to depend considerably on the TME and may represent therapy-induced hypoxia Zaman et al. On the other hand, high VEGFA levels could also be attributed to TP53 mutated lung tumors which correlated with improved efficacy of bevacizumab Schwaederlé et al. A currently identified alternative biomarker for bevacizumab-based chemotherapy combinations in patients with advanced NSCLC is CXCL In the analyzed sera of 40 advanced staged NSCLC patients therapy-induced decrease of CXCL16 levels correlated with prolonged OS compared with patients exhibiting only moderate decrement Shibata et al. However, confirming if any of these molecular markers indeed exhibit adequate predictive features necessitates further investigation. New aspects of processes which promote tumor angiogenesis, and a better understanding of the endothelium as driving force can help identify reliable biomarkers and overcome therapy failure in NSCLC. There are several mechanisms on both the cellular and environmental levels which can promote vessel formation in human tumors, many of which are not yet been completely elucidated. Although angiogenesis may represent the most important part of tumor vascularization, other processes that result in perfusion of the tumor tissue should be investigated in more detail and considered when designing new anti-angiogenic approaches in NSCLC. In the following part we summarize various levels of tumor vascularization that may represent new targets for vessel inhibition in NSCLC. All mentioned mechanisms are summarized in Figure 1. Figure 1. Mechanisms of tumor vascularization in NSCLC. Tumor vascularization in lung cancer can be promoted by various processes which overlap during cancer progression. TECs exhibit upregulated metabolism to enable high angiogenic activity which includes processes involved in proliferation cholesterol synthesis and glycolysis and processes that enable migration via ECM remodeling collagen synthesis. Potential targets involved in these pathways SQLE, PFKFB3, and ALDH18A1, respectively are considered to increase the angiogenic potential of TECs in NSCLC. Hypoxia and acidosis induced by high levels of lactate due to upregulated glycolysis constitute to a highly pro-angiogenic tumor environment. Angiogenesis stimulating factors VEGF, bFGF, PDGF, HIF-1α, tryptase, and MMPs are released by both, cancer cells and stromal cells, including fibroblasts, pericytes, tumor associated macrophages and ECs. Non-angiogenic processes constitute to tumor vascularization and are inaccessible for anti-angiogenic agents, thus contributing to therapy resistance. VM comprises the formation of tubular structures arising from cancer cells that gain endothelial like properties to maintain vascular supply during cancer progression. Another mechanism of cancer cells to persist in circulation is to grow along existing vasculature, which is referred to as vessel co-option. In this figure we summarized the various mechanism of tumor vascularization that should be considered when targeting the inhibition of tumor vessels in NSCLC. The endothelium is postulated to be a large contributor to the therapeutic efficacy of anti-angiogenic therapies, and therefore represents a possible source of therapy response or failure. It is well known that the process of angiogenesis is comprised of different EC phenotypes which execute distinct functions. During the elongation of the sprouting vessel VEGF-sensitive tip ECs migrate into avascular tissue regions, thus leading the proliferating trailing stalk ECs, which built up the growing vessel. Newly formed vasculature finally adapts a mature and quiescent phenotype referred to as phalanx ECs Carmeliet and Jain, ; Betz et al. The EC phenotypes involved are highly dynamic and can reprogram the gene expression to meet their current physiological requirements. However, the tumor endothelium was not studied in depth and a recent single-cell RNA sequencing scRNA-Seq study identified even more EC phenotypes from both healthy and tumor tissue from lung cancer samples as already known, indicating a much more complex phenotypic heterogeneity of the tumor vasculature than initially presumed Goveia et al. Interestingly, although phenotype proportions differed strongly between analyzed NSCLC patients, they collectively observed a low abundance of tip and proliferating TECs, which represent the main targets of traditional anti-angiogenic therapy. Furthermore, they identified a so-far-unknown tumor exclusive phenotype of activated postcapillary vein EC that upregulated features known from HEVs in inflamed tissues such as immunomodulatory factors and ribosomal proteins. The unexpected finding that activated and proliferating TECs only represent a minority of the pathological EC phenotypes found in NSCLC, allows us to reconsider currently used anti-angiogenic therapy as less of a vessel-inhibiting strategy, and more of a strategy to modulate the higher proportion of mature TECs into potent participants of tumor surveillance. In order to develop new angiogenesis-inhibiting therapies, the molecular differences between physiological and pathological ECs will need to be elaborated. Genetically TEC and NEC phenotypes significantly differ in gene expression affecting diverse cellular mechanisms such as proliferation, migration, inflammation, and angiogenesis Figure 2. Previous studies have shown that one key feature of TECs is a highly active metabolism, which permits pathological processes as increased proliferation and angiogenesis Cantelmo et al. Hyperglycolytic TECs subsequently release high amounts of lactate into the environment, which in turn, further stimulates EC proliferation and angiogenesis Annan et al. It could be demonstrated that inhibition of PFKFB3 resulted in improved drug efficacy and decreased metastatic events in tumor mouse models Cantelmo et al. Another study in xenograft NSCLC mouse models exhibited that PFKFB3 mRNA silencing in combination with docetaxel results in a chemoenhancing effect and increases anti-cancer efficacy compared with monotherapies alone Chowdhury et al. Furthermore, to sustain upregulated proliferative capacity, TECs exhibit elevated nucleotide biosynthesis including upstream pathways that are involved in serine and lipid synthesis Cantelmo et al. In addition, Lambrechts et al. Interestingly, c-MYC expression induces angiogenesis in combination with HIF-1α and VEGF Lee and Wu, and recruits tryptase positive mast cells into the tumor niche Soucek et al. Figure 2. The multifaced picture of TECs in NSCLC. TECs possess features that enable continuous angiogenic activity for progressing vascularization of the tumor. These features are ensured by genetical changes in the tumor endothelium that are triggered by diverse stimuli of the TME e. The stroma, consisting of various cells, promote angiogenesis by directly releasing signaling molecules into the adjacent tissue, thereby stimulating TECs. Fibroblasts and myeloid derived suppressor cells MDSCs activate angiogenesis by releasing VEGF and bFGF into the TME. Additionally, CSF-1 molecules, expressed by cancer cells, further recruit MDSCs into the tumor niche. Tumor associated macrophages TAMs can directly induce angiogenesis by releasing VEGF, bFGF, and PlGF, or indirectly by releasing matrix metalloproteinases MMPs which promote endothelial migration. Mast cells secrete tryptase TRYPT into the TME which stimulates EC proliferation and enables ECM remodeling. Furthermore, to facilitate enhanced angiogenesis, TECs upregulate the surface expression of angiogenic receptors as well as increase metabolic activity including energy and amino acid metabolism and the biosynthesis of nucleotides. In addition to the high angiogenic activity, TECs can directly suppress inflammatory responses by downregulation of inflammatory cytokines for immune cell recruitment CCL2, CCL8, and IL-6 , receptors required for immune cell homing ICAM or lymphocyte activation MHC I and MHC II which results in impaired immune cell trafficking and migration into the TME. In summary the complex interaction of tumor-protecting environmental conditions and the pathological features of TECs lead to a pro-angiogenic and immune suppressive TME in NSCLC. Focusing on endothelial metabolism in cancer, a recent study could identify at least two metabolic signatures which are highly upregulated in angiogenic endothelium and TECs. One for proliferation, which includes gene sets associated with biomass production e. These results educed two new possible metabolic targets to hamper tumor angiogenesis; aldehyde dehydrogenase 18 family member A1 ALDH18A1 , an enzyme essential for de novo biosynthesis of proline; and squalene epoxidase SQLE , the rate-limiting enzyme in cholesterol biosynthesis. Silencing of ALDH18A as well as SQLE impaired EC proliferation, migration and vessel sprouting in in vitro assays. Summarized, targeting endothelial metabolism in cancer is an interesting therapeutic option that could possibly assist an anti-angiogenic approach for treating NSCLC. Another key feature of TECs in lung cancer is the downregulation of inflammatory responses thus contributing to tumor-associated immune escape. Single-cell analysis of NSCLC samples identified the most downregulated genes of the tumor endothelium in connection to inflammation, which included CCL2, CCL18, and IL6, essential for immune cell recruitment; MHC I and II, essential for immune cell activation; and ICAM, required for immune cell homing Lambrechts et al. As the endothelium represents the primary connection between the immune system and tumor cells, these results indicate the important role of TECs in immunomodulatory processes that hamper anti-tumor immunity. Vessel normalization not only improves immune cell activation and infiltration, but is also suggested to enhance drug delivery to the tumor sites, thus improving its efficacy Allen et al. Additionally, combinational therapy of angiogenesis inhibitors and immunotherapy anti-PD-L1 in previous studies could elicit the formation of unique blood vessels in treated tumors that resemble HEVs typically found in lymphoid tissues, which implicated increased treatment efficacy Allen et al. HEVs can mediate immune cell adhesion and migration into the tumor, which may be important for bypassing TEC-induced immune escape Ager and May, In the already discussed scRNA-Seq study by Goveia et al. These remarkable observations indicate that TECs comprise the ability to transform into HEVs to promote immune cell infiltration into the tumor and induce a potent anti-tumor response. This extends the previous observations of favorable synergistic effects of immune therapy in combination with angiogenesis inhibitors in NSCLC, especially when it results in HEV formation. Furthermore, direct induction of HEV formation could be a promising new strategy in anti-angiogenic approaches that may attain great clinical importance. However, currently there are no reliable biomarkers to track the process of vessel normalization or HEV formation in NSCLC which could help to predict and optimize this new treatment strategy. As mentioned above, in some cases tumor vascularization can be facilitated by non-ECs which adapt certain properties to sustain access to the circulation, which may support anti-angiogenic drug resistance. During tumor progression, processes that lead to vascularization of the malignant tissue can vary locally as well as temporarily and involve angiogenic as well as non-angiogenic mechanisms even in the same lesion Bridgeman et al. In lung tumors, where non-angiogenic tumor growth occurs most commonly, previous studies primarily located non-angiogenic processes in the tumor periphery, whereas angiogenesis is typically localized in the hypoxic tumor core Pezzella et al. Here, we briefly discuss the impact of non-angiogenic processes in NSCLC on anti-angiogenic drug efficacy based on previous studies. VEGF-A inhibition using bevacizumab failed to inhibit VM in breast cancer cells in vitro , furthermore, sunitinib, a multi targeting anti-VEGFR inhibitor, even promoted VM in breast cancer mouse models Dey et al. Additionally it could be demonstrated that VM in NSCLC depends on expression of Sema4D and its receptor plexinB1 which activate RhoA and downstream ROCK, comprising an already known angiogenesis-promoting process in tumors Basile et al. Although the role of VM in NSCLC is not fully understood, previous observations suggest that it may contribute to anti-angiogenic therapy failure and may serve as an option to treat aggressive lung tumors. Vessel co-option on the other hand is a common phenomenon especially observed in lung metastases when tumor cells start to invade perivascular tissues Jensen, Anti-angiogenic therapy with sunitinib could induce a switch from angiogenic vessel formation to vessel co-option in a lung metastatic mouse model, which ultimately resulted in sunitinib resistance Bridgeman et al. Unfortunately, regulative mechanisms of vessel co-option in human tumors remain unknown in large part, however, predicting the occurrence of either VM or vessel co-option could be a useful tactic to prevent anti-angiogenic drug resistance in some patients. According to these and other results, it could be confirmed that non-angiogenic tumors contribute to anti-angiogenic therapy resistance which reveals the undoubted importance of targeting both angiogenic, but also non-angiogenic vessel growth to treat NSCLC Donnem et al. Increasing knowledge of the physiological processes of tumor vascularization in addition to traditional angiogenesis has enlightened a variety of adaptive mechanisms which can promote anti-angiogenic therapy resistances. This awareness fortifies the necessity for alternative anti-angiogenic agents besides traditional anti-VEGF therapy. As previously examined, tumor angiogenesis depends on upregulated metabolic activity e. Cholesterol not only represents a fundamental structural component of cell membranes and serves as precursor for several steroid hormones, it is also crucial for membrane function and angiogenic signaling, making it a favorable target for tumor vessel inhibition Lyu et al. Inhibition of intracellular cholesterol trafficking with anti-inflammatory drug chepharantine was shown to hamper angiogenesis and tumor growth in lung cancer xenograft mice while improving anti-tumor activity of standard chemotherapeutics Lyu et al. Another study has shown that pharmacological lowering of intracellular cholesterol levels with pitavastatin could reduce growth and migration and induced apoptosis in human lung tumor-associated ECs in vitro Hu et al. In vivo experiments using lung cancer xenograft mice exhibited that pitavastatin-treatment could completely arrest tumor growth in these animals when combined with cisplatin and delayed tumor growth and impaired angiogenesis in cisplatin-resistant mouse models. Another potential angiogenic target for cancer treatment is tie1. While the second tie receptor, tie2, is well characterized as a regulator during late stages of angiogenesis e. As tie1 is also upregulated in intratumoral vasculature, its deletion on ECs successfully produced a potent anti-angiogenic effect in different cancers Kaipainen et al. In fact, EC-specific deletion of tie1 in lung carcinoma and melanoma mouse models resulted in delayed cancer growth, predominantly in late-stage tumors La Porta et al. Furthermore, it inhibited neovessel sprouting and a reduced intratumoral vessel density, while the remaining mature vasculature became strongly normalized, which limited further metastatic formation. These findings, and the fact that tie1 expression is increased in angiogenic endothelium compared with resting vasculature, presents tie1 as a highly potent angiogenic target, especially in the treatment of advanced staged NSCLC. Another considerable strategy of anti-angiogenic therapy could include targeting micro RNAs miRNAs as they represent a new paradigm in molecular cancer therapy. The impact of miRNAs in post-transcriptional regulation has already been associated with pathways involved in cancer and vascular disease as summarized in Sun et al. The following studies evaluated the potential role of specific angiogenesis-related miRNAs as targets in lung cancer. Hsu et al. observed that miRa, a micro RNA known to be hypoxia-associated, was overexpressed in exosomes of oxygen depleted CL lung cancer cells Hsu et al. Furthermore, these cancer-cell derived exosomes could induce angiogenesis via HIF-1α signaling in vitro when internalized by HUVECs. Additionally, miRa transfection increased permeability and transendothelial migration of cancer cells in vitro by downregulation of the tight junction protein ZO-1 and stimulated neovascularization and tumor growth in vivo in CL xenograft mice, proposing it to be an appealing target for anti-angiogenic therapy. Upregulation of miR in squamous lung cancer cells in vitro on the other hand could be associated with impaired VEGF expression and hampered migration and invasion, thereby facilitating a tumor-suppressive function. Additionally, overexpression of miR in HUVECs was observed to inhibit tube formation and reduced the expression of VEGF, which hampered their angiogenesis activity in vitro Liu et al. As it is an essential process during vessel growth, targeting ECM remodeling may also be an interesting approach to inhibit tumor angiogenesis in NSCLC. The most prominent enzymes involved in this process are matrix-metalloporoteinases MMPs which are inhibited under physiological conditions by tissue inhibitors of metalloproteinases TIMPs. miRb could be identified as a promotor of MMP-2 activity and invasion of NSCLC cancer cells in vitro by downregulation of TIMP Additionally, it could be observed that miRb was significantly upregulated in tumor tissue of NSCLC patients with vascular cancer cell invasion Hirono et al. According to these findings, targeting miRb could be a strategy to impede angiogenesis and cancer cell invasion in lung cancer. Uribesalgo et al. suggested targeting the apelin signaling pathway to inhibit tumor vessel formation in lung cancer Uribesalgo et al. Apelin is a conserved peptide involved in developmental angiogenesis and is also upregulated in ECs within the TME. Previous studies could associate high apelin levels with a poor clinical outcome in patients with NSCLC Györffy et al. In murine lung cancer models, apelin knockout reduced tumor burden and prolonged survival by inhibiting VEGF, TGF-β1, and TNF-α and simultaneously decreased MDSC infiltration in the TME Uribesalgo et al. The combination of pharmacological inhibition of apelin with the anti-angiogenic drug sunitinib in lung cancer and mammary cancer mouse models, significantly delayed tumor growth and could almost double the survival, even in the KRAS driven or p53 mutated tumors, when compared with sunitinib treatment alone. Finally, apelin loss also reduced vessel density and prevented sunitinib-induced hypoxia and poor vessel structure in the TME. Conclusively, apelin inhibition may provide a potent synergistic anti-tumor effect when combined with anti-angiogenic agents, while, and most importantly, avoiding therapy-induced hypoxia of the TME, thus decreasing the chance of metastases, and bypassing potential therapy resistances. Single-target anti-angiogenic agents have already shown their limitations in clinical settings Jayson et al. Even in combination with other therapy approaches like standard chemotherapy or immune therapy, treatment success remains largely marginal. Targeting several pro-angiogenic molecules with recombinant fusion proteins could therefore increase the anti-angiogenic effect of such therapies. Zhang et al. When injected into lung cancer mouse models, autologous generated anti-peptibody antibodies inhibited tumor progression and angiogenesis and decreased expression of bFGF, VEGFA and PDGF in the tumor tissue. Targeting angiogenesis with fusion proteins exhibited potent anti-tumor efficacy in murine models and may represent a new approach for vessel inhibition in NSCLC, especially in combination with other therapy agents aimed at important angiogenic factors, previously discussed potential TEC specific markers or cellular mechanisms Table 2. The instability of tumor vessels due to morphological abnormalities e. Although anti-angiogenic therapy can temporarily restore tissue perfusion and drug delivery by vascular normalization, treatment withdrawal often results in vessel hyper-permeability and can even induce a rebound effect of tumor angiogenesis Yang et al. As continuous inhibition of angiogenesis remains difficult to implement for health or economic reasons, an alternative or more independent delivery system of anti-angiogenic agents could help to overcome these issues. Nanomaterials have become an emerging field in cancer therapy in recent years, as their unique molecular properties make them suitable targeted drug delivery-systems. Physiochemically, these nanoparticles match the size of inter-endothelial junctions of blood vessels in the TME and therefore increase permeation and retention EPR resulting in a passive drug delivery Chauhan and Jain, Nanomaterials such as liposomes or nanotube carbon structures are used to deliver anti-angiogenic agents and improve drug specificity while reducing cytotoxic side effects, drug clearance and resistance mechanisms in the treatment of NSCLC Seshadri and Ramamurthi, In the past, studies using biodegradable polymers as nanocarriers to deliver chemotherapeutics and targeted drugs exhibited significant anti-tumor efficacy in vitro and in vivo. For example, paclitaxel encapsulated aldehyde polyethylene glycol-polylactide PEG-PLGA conjugated to a VEGFR2-inhibiting peptide showed increased internalization in HUVECs in vitro as well as potent activity against breast cancer models in vivo Yu et al. Although there are several peptide motifs that are suggested to target tumor endothelium such as RGD or NGR which can bind integrin heterodimers CD51 and CD61, or aminopeptidase N, respectively, their targeting with nanomaterial is not yet applied for treating NSCLC Sakurai et al. Furthermore, non-angiogenic mechanisms such as VM or vessel co-option could also represent possible targets for nanomaterial-based therapy as the EPR effect of such molecules could help to overcome delivery and infiltration issues of traditional cancer therapeutics. However, nanotherapeutics may provide a new potential anti-angiogenic therapeutical approach, but as already discussed, there is still a need for more specific biomarkers to exclusively target tumor vasculature in an organ specific manner. Taking this into consideration, chimeric antigen receptor CAR T-cell therapy, which serves as personalized immune therapy using autologous T-lymphocytes, engineered to target specific antigens present in a tumor, could be used to exclusively eliminate TECs without damaging healthy vasculature. The therapy failure can, at least in part, be attributed to the impaired accessibility of the tumor mass due to dysfunctional vasculature and immunosuppressive conditions in the TME. Targeting tumor vessels directly with CAR T-cells could therefore be a good strategy to overcome these issues, which at best, can normalize the defective vasculature and improve drug efficacy in combinational therapy settings. In a recent study Xie et al. Injected EIIIB-targeting CAR T-cells could delay tumor growth and improve survival in immunocompetent mouse models harboring aggressive melanoma, whereas colorectal cancer mouse models did not respond to the treatment. Here, the expression levels of EIIIB in the different tissues had impact on the therapy outcome which again highlights the importance of organ specific vascular markers as well as the impact of organ specific angiogenic activity when targeting tumor vessel formation. Other studies investigated the anti-angiogenic efficacy of TEM8-specific CAR T cells in solid cancer mouse models. TEM8 is one of the first discovered TEC markers and represents a promising target in anti-angiogenic therapy strategies St Croix et al. In , a study reported that TEM8-specific CAR T-cells could improve survival and significantly decreased vascularization in triple negative breast cancer mouse models and induced tumor regression in mice with lung metastases Byrd et al. A more recent study, however, observed contrasting results where TEM8-sepcific CAR T-cells triggered high toxicity and induced inflammation in lung and spleen when injected into healthy mice Petrovic et al. It is suggested that the engineered T-cells cross-reacted with other antigens or targeted TEM8 in healthy tissues, although it is normally expressed at a much lower quantity compared with pathological levels. However, both processes resulted in severe toxicity in vivo and again emphasize the need for more adequate, highly specific tumor-vessel exclusive markers that can be targeted with either CAR T-cells or other previously discussed inhibiting molecules. So far, the main obstacles of anti-angiogenic therapy in NSCLC are evading- or intrinsic resistance mechanisms which still remain elusive. We have discussed a wide array of possible therapies and therapy systems that could improve anti-angiogenic efficacy when combined with standard treatment. The principal goal would be to expand the therapeutical effect of angiogenesis-inhibiting drugs on vessel normalization and render the tumor more vulnerable to additional agents such as chemotherapy or immunotherapy. In a recent study, Hosaka et al. could show that dual angiogenesis inhibition could sensitize resistant off-target tumors to therapy. Therefore they created mouse models of breast cancer or fibrosarcoma, both resistant to anti-VEGF and anti-PDGF treatment due to increased tumor associated expression of bFGF, a molecule which modulates the vasculature via pericyte recruitment in a PDGF-dependent process Hosaka et al. Neither anti-VEGF nor anti-PDGF monotherapy had a significant anti-tumor effect on bFGF-positive tumors, but the combination of both agents produced a superior benefit, inhibiting cancer growth by suppressing proliferation and triggering apoptosis of tumor cells. Interestingly, even the pan-blocking of FGF-receptors did not yield a comparable benefit. To explain this unexpected effect, angiogenesis has to be considered as an interacting network of various signaling pathways which cannot be disrupted by blocking a single molecule. These findings demonstrate that the disruption of interacting angiogenic pathways by simultaneously targeting multiple angiogenic factors can provoke a highly potent anti-tumor effect which is able to circumvent mechanisms of therapy resistance, and thus should be considered as new approach to improve neovessel inhibition in cancer. Angiogenesis is a main therapeutic concept in oncology, especially in NSCLC, where three approved agents are available in combination with chemotherapy or immunotherapy. Increasing knowledge in angiogenic processes and non-angiogenic processes that contribute to tumor vascularization, provide precise targets for novel therapy strategies and pave the way for developing new anti-angiogenic treatment concepts that target e. These therapeutic concepts need to be evaluated for synergistic effects as, in our view, modern anti-angiogenesis represents the concept of shaping the TME rather than being a direct anti-tumor therapy itself. However, these therapeutic strategies are very promising in preclinical setting and the translation into a clinical setting is not only warranted but highly desired. Furthermore, a new horizon of targeted and functional TEC characterization was opened by scRNA-Seq studies, which proved that the tumor vasculature is highly heterogenous and differs from the normal adjacent vasculature more than primarily assumed in terms of metabolic activity, immune suppression and heterogeneity for example. In addition, new synergistic effects of TECs in their role of immunomodulation were identified and induction of HEV formation for immune priming is suggested to be a new therapeutic strategy. Next the organ specific context of the vasculature plays an important role and has to be further studied for better therapy allocation. In conclusion the concept and goal of anti-angiogenesis in NSCLC in the future can be reshaped by abolishing the traditional vessel priming concept and moving toward a side specific molding of the TME, using the tumor vasculature as a tool, like a trojan horse. SD, HH, EN, AP, and DW developed the concept of the review. SD, HH, and EN drafted the review. DW and AP corrected and reviewed the review. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Adighibe, O. Is nonangiogenesis a novel pathway for cancer progression? A study using 3-dimensional tumour reconstructions. Cancer 94, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ager, A. Understanding high endothelial venules: lessons for cancer immunology. Oncoimmunology 4:e Aguayo, A. Clinical relevance of Flt1 and Tie1 angiogenesis receptors expression in B-cell chronic lymphocytic leukemia CLL. Leukemia Res. CrossRef Full Text Google Scholar. Allen, E. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Alshangiti, A. Antiangiogenic therapies in non-small-cell lung cancer. Annan, D. Carbonic anhydrase 2 CAII supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Auf, G. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Augustin, H. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Cell Biol. Augustine, R. Therapeutic angiogenesis: from conventional approaches to recent nanotechnology-based interventions. C Mater. Babina, I. Advances and challenges in targeting FGFR signalling in cancer. Cancer 17, — Basile, J. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Bergers, G. Tumorigenesis and the angiogenic switch. Cancer 3, — Modes of resistance to anti-angiogenic therapy. Cancer 8, — Bertolini, F. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Cancer 6, — Betz, C. Cell behaviors and dynamics during angiogenesis. Development , — Bittner, M. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature , — de Bock, K. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell , — Bridgeman, V. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. Bruning, U. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. Byrd, T. Cancer Res. |
| Why Antiangiogenesis Fails | Harvard Medical School | Iyer, S. Role of placenta growth factor in cardiovascular health. Trends Cardiovasc. Beck, H. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. Donnini, S. Expression and localization of placenta growth factor and PlGF receptors in human meningiomas. Lacal, P. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. Nonclassic endogenous novel regulators of angiogenesis. Byrne, A. Angiogenic and cell survival functions of vascular endothelial growth factor VEGF. Barleon, B. Migration of human monocytes in response to vascular endothelial growth factor VEGF is mediated via the VEGF receptor flt Blood 87 , — Angiogenesis 9 , — The biology of VEGF and its receptors. Ishida, A. Expression of vascular endothelial growth factor receptors in smooth muscle cells. Ghosh, S. High levels of vascular endothelial growth factor and its receptors VEGFR-1, VEGFR-2, neuropilin-1 are associated with worse outcome in breast cancer. Ceci, C. Ioannidou, E. Angiogenesis and anti-angiogenic treatment in prostate cancer: mechanisms of action and molecular targets. Simons, M. Mechanisms and regulation of endothelial VEGF receptor signalling. Molhoek, K. VEGFR-2 expression in human melanoma: revised assessment. Spannuth, W. Functional significance of VEGFR-2 on ovarian cancer cells. Capp, C. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20 , — Modi, S. Padró, T. Overexpression of vascular endothelial growth factor VEGF and its cellular receptor KDR VEGFR-2 in the bone marrow of patients with acute myeloid leukemia. Leukemia 16 , — Sun, W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. Valtola, R. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Saintigny, P. Vascular endothelial growth factor-C and its receptor VEGFR-3 in non-small-cell lung cancer: concurrent expression in cancer cells from primary tumour and metastatic lymph node. Lung Cancer 58 , — Yonemura, Y. Lymphangiogenesis and the vascular endothelial growth factor receptor VEGFR -3 in gastric cancer. Cancer 37 , — Su, J. Cancer 96 , — Simiantonaki, N. Google Scholar. Goel, H. VEGF targets the tumour cell. Cancer 13 , — Wang, H. PLoS ONE 7 , e Manzat Saplacan, R. The role of PDGFs and PDGFRs in colorectal cancer. Mediators Inflamm. Kalra, K. Cell Dev. Balamurugan, K. Cenciarelli, C. PDGFRα depletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals. Oncotarget 7 , — Chabot, V. Stem Cell Res. Li, H. Development of monoclonal anti-PDGF-CC antibodies as tools for investigating human tissue expression and for blocking PDGF-CC induced PDGFRα signalling in vivo. PLoS ONE 13 , e Dardik, A. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1α. Muratoglu, S. Low density lipoprotein receptor-related protein 1 LRP1 forms a signaling complex with platelet-derived growth factor receptor-β in endosomes and regulates activation of the MAPK pathway. Wang, J. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. Cancer Res. Li, M. Integrins as attractive targets for cancer therapeutics. Acta Pharm. B 11 , — Zou, X. Redundant angiogenic signaling and tumor drug resistance. Lee, C. Platelet-derived growth factor-C and -D in the cardiovascular system and diseases. Berthod, F. Spontaneous fibroblast-derived pericyte recruitment in a human tissue-engineered angiogenesis model in vitro. The role of pericytes in angiogenesis. Chatterjee, S. Pericyte-endothelial cell interaction: a survival mechanism for the tumor vasculature. Cell Adh. Luk, K. Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy. Cavalcanti, E. PDGFRα expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors. Cancer Biol. Burger, R. Overview of anti-angiogenic agents in development for ovarian cancer. Raica, M. Pharmaceuticals 3 , — Heindryckx, F. Targeting the tumor stroma in hepatocellular carcinoma. World J. Cornellà, H. Molecular pathogenesis of hepatocellular carcinoma. Brahmi, M. Expression and prognostic significance of PDGF ligands and receptors across soft tissue sarcomas. ESMO Open 6 , Rao, L. HB-EGF-EGFR signaling in bone marrow endothelial cells mediates angiogenesis associated with multiple myeloma. Cancers 12 , Hu, L. Dual target inhibitors based on EGFR: promising anticancer agents for the treatment of cancers Larsen, A. Targeting EGFR and VEGF R pathway cross-talk in tumor survival and angiogenesis. Holbro, T. ErbB receptors: directing key signaling networks throughout life. Ellis, L. Epidermal growth factor receptor in tumor angiogenesis. North Am. De Luca, A. The role of the EGFR signaling in tumor microenvironment. Albadari, N. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Bos, R. Hypoxia-inducible factor-1α is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology 46 , 31—36 Salomon, D. Epidermal growth factor-related peptides and their receptors in human malignancies. Yu, H. Poor response to erlotinib in patients with tumors containing baseline EGFR TM mutations found by routine clinical molecular testing. Raj, S. Cancer 21 , 31 Acevedo, V. Paths of FGFR-driven tumorigenesis. Cell Cycle 8 , — Chen, M. Progress in research on the role of FGF in the formation and treatment of corneal neovascularization. Montesano, R. Basic fibroblast growth factor induces angiogenesis in vitro. USA 83 , — Giacomini, A. Hui, Q. FGF family: from drug development to clinical application. Presta, M. Cytokine Growth Factor Rev. Fons, P. Katoh, M. FGF receptors: cancer biology and therapeutics. Kopetz, S. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. Batchelor, T. AZD, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11 , 83—95 Cancer genomics and genetics of FGFR2 Review. Fibroblast growth factor signalling: from development to cancer. Cancer 10 , — Greulich, H. Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol. Cross, M. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. García-Caballero, M. Angioprevention of urologic cancers by plant-derived foods. Pharmaceutics 14 , Aviles, R. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor FGF-2 : Clinical angiogenesis using FGF Vasudev, N. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17 , — Ding, S. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood , — Bonnans, C. Remodelling the extracellular matrix in development and disease. Mulcahy, E. Nakamura, T. The discovery of hepatocyte growth factor HGF and its significance for cell biology, life sciences and clinical medicine. B: Phys. Bottaro, D. Identification of the hepatocyte growth factor receptor as the c- met proto-oncogene product. Science , — Dean, M. The human met oncogene is related to the tyrosine kinase oncogenes. Ono, K. Circulation 95 , — Cai, W. Mechanisms of hepatocyte growth factor—induced retinal endothelial cell migration and growth. Ankoma-Sey, V. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene 17 , — Nagashima, M. Hepatocyte growth factor HGF , HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. Hughes, P. In vitro and in vivo activity of AMG , a potent and selective MET kinase inhibitor, in MET-dependent cancer models. Leung, E. Oncogene 36 , — Kuang, W. Hartmann, S. Demuth, C. Increased PD-L1 expression in erlotinib-resistant NSCLC cells with MET gene amplification is reversed upon MET-TKI treatment. Oncotarget 8 , — Kwon, M. Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus-negative tonsillar squamous cell carcinoma and their prognostic significances. Miranda, O. Cancers 10 , Wang, Q. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Wu, J. Prostate 76 , — Imura, Y. Cancer Sci. Birchmeier, C. Met, metastasis, motility and more. Zhang, Y. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Cancer 17 , 45 Scalia, P. The IGF-II-insulin receptor isoform-A autocrine signal in cancer: actionable perspectives. Bach, L. Endothelial cells and the IGF system. Clemmons, D. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. van Beijnum, J. Insulin-like growth factor axis targeting in cancer and tumour angiogenesis—the missing link: IGF signaling in tumor angiogenesis. Chantelau, E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Wilkinson-Berka, J. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Higashi, Y. Aging, atherosclerosis, and IGF A: Biol. Hellstrom, A. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. USA 98 , — Smith, L. Pathogenesis of retinopathy of prematurity. Acta Paediatr. Moschos, S. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63 , — Sachdev, D. The IGF system and breast cancer. Samani, A. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Baserga, R. The IGF-1 receptor in cancer biology. Azar, W. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology , — Png, K. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Liu, B. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene 26 , — Wu, M. TGF-β superfamily signaling in embryonic development and homeostasis. Cell 16 , — Yang, Y. The role of TGF-β signaling pathways in cancer and its potential as a therapeutic target. Based Complement Altern. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb. Santoro, R. TAK-ing aim at chemoresistance: the emerging role of MAP3K7 as a target for cancer therapy. Colak, S. Targeting TGF-β signaling in cancer. Trends Cancer 3 , 56—71 Platten, M. Malignant glioma biology: Role for TGF-β in growth, motility, angiogenesis, and immune escape. Sabbadini, F. The multifaceted role of TGF-β in gastrointestinal tumors. Massagué, J. TGFβ signalling in context. Horiguchi, K. Role of Ras signaling in the induction of snail by transforming growth factor-β. Korc, M. Role of growth factors in pancreatic cancer. Nolan-Stevaux, O. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. Budi, E. Enhanced TGF-β signaling contributes to the insulin-induced angiogenic responses of endothelial cells. iScience 11 , — Darland, D. Angiogenesis 4 , 11—20 Huynh, L. A perspective on the development of TGF-β inhibitors for cancer treatment. Biomolecules 9 , Katz, L. TGF-β signaling in liver and gastrointestinal cancers. Tsubakihara, Y. Epithelial-mesenchymal transition and metastasis under the control of transforming growth factor β. Fu, M. Multifunctional regulatory protein connective tissue growth factor CTGF : a potential therapeutic target for diverse diseases. B 12 , — Pepper, M. Transforming growth factor-β: vasculogenesis, angiogenesis, and vessel wall integrity. Fang, L. TGF-β1 induces VEGF expression in human granulosa-lutein cells: a potential mechanism for the pathogenesis of ovarian hyperstimulation syndrome. Oncogenesis 9 , 76 Melisi, D. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. Zhang, H. TGFβ signaling in pancreatic ductal adenocarcinoma. Tumor Biol. Modulating TAK1 expression inhibits YAP and TAZ oncogenic functions in pancreatic cancer. Gladilin, E. TGFβ-induced cytoskeletal remodeling mediates elevation of cell stiffness and invasiveness in NSCLC. Mazzocca, A. Inhibition of transforming growth factor β receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology 50 , — Bhagyaraj, E. TGF-β induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell Cycle 18 , — Carcinogenesis 39 , — Chiechi, A. Role of TGF-β in breast cancer bone metastases. Masoud, G. HIF-1α pathway: role, regulation and intervention for cancer therapy. B 5 , — HIFs, angiogenesis, and cancer. Konisti, S. Hypoxia—a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Ahluwalia, A. Critical role of hypoxia sensor—HIF-1 in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Tanaka, T. Angiogenesis and hypoxia in the kidney. Chen, L. Hypoxia and angiogenesis: regulation of hypoxia-inducible factors via novel binding factors. Ikeda, H. Targeting hypoxia-inducible factor 1 HIF-1 signaling with natural products toward cancer chemotherapy. Pugh, C. Regulation of angiogenesis by hypoxia: role of the HIF system. Semenza, G. Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization. Vleugel, M. Differential prognostic impact of hypoxia induced and diffuse HIF-1 expression in invasive breast cancer. Dales, J. Overexpression of hypoxia-inducible factor HIF-1α predicts early relapse in breast cancer: retrospective study in a series of patients. Yatabe, N. HIFmediated activation of telomerase in cervical cancer cells. Oncogene 23 , — Pezzuto, A. A close relationship between HIF-1α expression and bone metastases in advanced NSCLC, a retrospective analysis. Oncotarget 10 , — Jackson, A. HIF, hypoxia and the role of angiogenesis in non-small cell lung cancer. Targets 14 , — Liu, K. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. Zheng, S. Prognostic significance of HIF-1α expression in hepatocellular carcinoma: a meta-analysis. PLoS ONE 8 , e Targeting HIF-1 for cancer therapy. Schöning, J. Rohwer, N. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Zhang, Q. Cell , 37—57 Iosef, C. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Lung Cell. Sakamoto, K. Constitutive NF-κB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Huang, S. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20 , — Davis, S. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Saharinen, P. Therapeutic targeting of the angiopoietin-TIE pathway. Partanen, J. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Fagiani, E. Angiopoietins in angiogenesis. Shim, W. Angiopoietin: a TIE d balance in tumor angiogenesis. Cancer Res 5 , — Gillen, J. Angiopoietin-1 and angiopoietin-2 inhibitors: clinical development. Eklund, L. Angiopoietin—Tie signalling in the cardiovascular and lymphatic systems. Takahama, M. Enhanced expression of Tie2, its ligand angiopoietin-1, vascular endothelial growth factor, and CD31 in human non-small cell lung carcinomas1. Tangkeangsirisin, W. PC Cell-derived growth factor mediates tamoxifen resistance and promotes tumor growth of human breast cancer cells. Cancer Res 64 , — Shirakawa, K. Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Martoglio, A. Changes in tumorigenesis- and angiogenesis-related gene transcript abundance profiles in ovarian cancer detected by tailored high density cDNA arrays. Ding, H. Expression and hypoxic regulation of angiopoietins in human astrocytomas. Stratmann, A. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Udani, V. Differential expression of angiopoietin-1 and angiopoietin-2 may enhance recruitment of bone marrow-derived endothelial precursor cells into brain tumors. Thurston, G. The complex role of angiopoietin-2 in the angiopoietin-Tie signaling pathway. Angiopoietin signaling in the vasculature. Bolós, V. Notch signaling in development and cancer. Ranganathan, P. Notch signalling in solid tumours: a little bit of everything but not all the time. Cancer 11 , — Takeshita, K. Critical role of endothelial notch1 signaling in postnatal Angiogenesis. Blanco, R. VEGF and Notch in tip and stalk cell selection. Radtke, F. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Gustafsson, M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Cell 9 , — Sainson, R. Diez, H. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Patel, N. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Reedijk, M. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Tschaharganeh, D. Yes-associated protein up-regulates Jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology , — Yousif, N. Che, L. Jagged 1 is a major Notch ligand along cholangiocarcinoma development in mice and humans. Oncogenesis 5 , e Bellon, M. JAG1 overexpression contributes to Notch1 signaling and the migration of HTLVtransformed ATL cells. Sethi, N. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19 , — Sjölund, J. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. Schneider, M. Inhibition of Delta-induced Notch signaling using fucose analogs. Sahlgren, C. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Surawska, H. The role of ephrins and Eph receptors in cancer. Zozulya, S. Eph family receptors as therapeutic targets. Zhang, J. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. Batlle, E. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Pasquale, E. Eph receptor signalling casts a wide net on cell behaviour. Eph-ephrin bidirectional signaling in physiology and disease. Cell , 38—52 Du, E. Yuan, C. Overexpression of ephrinB2 in stem cells from apical papilla accelerates angiogenesis. You, C. Yang, D. Angiogenesis 19 , — Pierscianek, D. Study of angiogenic signaling pathways in hemangioblastoma. Neuropathology 37 , 3—11 Avraamides, C. Integrins in angiogenesis and lymphangiogenesis. Bergonzini, C. Targeting integrins for cancer therapy - disappointments and opportunities. Takada, Y. The integrins. Genome Biol. Hynes, R. Mezu-Ndubuisi, O. The role of integrins in inflammation and angiogenesis. Huveneers, S. Adhesion signaling — crosstalk between integrins, Src and Rho. Cell Sci. Łasiñska, I. Integrins as a new target for cancer treatment. Anticancer Agents Med. Trikha, M. CNTO 95, a fully human monoclonal antibody that inhibits? αv integrins, has antitumor and antiangiogenic activityin vivo. Mitjans, F. In vivo therapy of malignant melanoma by means of antagonists of αv integrins. Cancer 87 , — Khalili, P. A non—RGD-based integrin binding peptide ATN blocks breast cancer growth and metastasis in vivo. Garmy-Susini, B. Integrin α4β1—VCAM-1—mediated adhesion between endothelial and mural cells is required for blood vessel maturation. Kim, S. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Vlahakis, N. Integrin α9β1 directly binds to vascular endothelial growth factor VEGF -A and contributes to VEGF-A-induced angiogenesis. Rooprai, H. The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Cancer 82 , 52—55 Laronha, H. Structure and function of human matrix metalloproteinases. Cells 9 , The authors note that their findings in animal models need to be validated in controlled clinical trials in human patients. Support for this study includes National Institutes of Health grants CA, T32 DK, CA, CA, CA, CA, CA, CA and CA Adapted from a Mass General news release. News Topic Menu News Topics Research Awards and Achievements Care Delivery HMS Community Education Stay Up to Date. First Name. Last Name. Email Address. Which publications would you like to receive? Harvard Medicine magazine monthly. Harvard Medicine News weekly. On the Brain quarterly. Why Antiangiogenesis Fails Team finds possible mechanism behind resistance to cancer treatment. By SUE McGREEVEY October 12, Research. Image; iStock. The Surprisingly Simple Recipe for Starting to Grow a Limb February 5, Study illuminates development, could inform limb regeneration efforts. Uncovering New Drivers of Heart Disease, Brain Vessel Disorders February 7, How genetic changes in cells that line blood vessels fuel cardiac disease, brain vessel…. |
| Top bar navigation | Viewpoint Collections In-Press Preview Commentaries Research Letters Letters to the Editor Editorials Viewpoint JCI This Month Top read articles Clinical Medicine. Importantly, as more and more promising biomarkers are uncovered, a further challenge will be to standardise methods of biomarker assessment across centres so that they can be validated prospectively and, eventually, utilised routinely. It has been combined with paclitaxel, carboplatin and liposomal doxorubicin in phase III trials [ 23 ]. In tumor tissues, an abnormal acidic pH environment may result from a variety of factors, including hypoxia, accumulation of acidic metabolites, and so on. Tong RT et al Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. GC-MS analyses of the water and methanol leaf extracts from N. |
| Angiogenesis and Angiogenesis Inhibitors to Treat Cancer | nintendoswitch.info | First isolated Antioxidant supplements for immune support a proteolytic digestion Resilience Anti-angiogendsis hepatocyte growth reesarch HGFNK4 is a novel anti-tumor Bad breath through its Ribose and sports performance activities Angi-angiogenesis HGF antagonism and anti-angiogenesis. Table Ressearch. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. See related article by Tong researchh colleagues, Cancer Res ;—6. Recent clinical trials in hepatocellular carcinoma have shown that the addition of antiangiogenic therapy to immunotherapy dramatically extends OS compared with the established standard of care Despite the promising prospect that cationic liposomes presented with several studies in clinical trials, the low transfection efficiency and side effects, including toxicity, are the primary obstacles preventing its widespread use 54 — |
Anti-angiogenesis research -
To develop better anti-angiogenic therapies, it will be vital for new anti-angiogenic strategies to be tested in models that more accurately reflect different disease stages.
In addition, there are a growing number of studies demonstrating that resistance to VEGF-targeted agents might be overcome by targeting a second pathway.
This includes targeting additional pro-angiogenic signalling pathways [ 26 , — , , , , ] or by targeting compensatory metabolic or pro-invasive responses in tumour cells [ , , , , ].
These studies are vital and should allow the design of rationale combination strategies that could be tested in the clinic.
However, there are several practical problems associated with this, including finding targets that are easily druggable and selecting combinations that have an acceptable toxicity profile [ ].
A consideration of these practicalities at the preclinical phase may accelerate the selection of new strategies that can be practically and rapidly translated to the clinic.
As we have seen, the biology determining response and resistance to anti-angiogenic therapy is complex. It is perhaps therefore unsurprising that predictive biomarkers for this class of agent remain elusive. To identify which patients will benefit from these therapies, mechanism-driven biomarkers are required that can account for the dynamic and complex underlying biology.
Importantly, as more and more promising biomarkers are uncovered, a further challenge will be to standardise methods of biomarker assessment across centres so that they can be validated prospectively and, eventually, utilised routinely.
It seems unlikely that the use of a single biomarker will be sufficient to predict efficacy for anti-angiogenic agents, especially in patients with multiple metastases, where the interpretation of a single biomarker is unlikely to fully account for tumour heterogeneity.
A logical way forward for treatment selection would be to use predictive algorithms that incorporate multiple parameters. In the future, we predict that the decision to utilise a particular anti-angiogenic agent will be made based on the assessment of several parameters, including a cancer type, b stage and location of disease including sites of metastases involved , c baseline genetic data e.
germline SNPs, d circulating markers acquired at baseline and during therapy, and e functional imaging data acquired both at baseline and during therapy. Moreover, in a world where multiple targeted agents are now potentially available for tailored treatment, the decision to use anti-angiogenic therapy will need to be weighed against the use of other potentially effective treatment options for each patient.
Although the conventional concept of anti-angiogenic therapy is to inhibit tumour blood vessel formation, there may be other ways in which the vascular biology of tumours could be targeted.
Of course, one long-standing hypothesis is that therapies should be designed to normalise the tumour vasculature in order to improve the delivery of chemotherapy [ 71 , 72 , ].
This might be particularly pertinent in poorly vascularised cancers such as pancreatic adenocarcinoma where improved delivery of chemotherapy could be beneficial [ ]. Moreover, vascular normalisation may have additional beneficial effects for controlling oedema or tumour oxygenation [ 74 , 75 ].
In addition, it is now known that blood vessels are not merely passive conduits for the delivery of oxygen and nutrients.
Furthermore, two recent studies showed that endothelial cells can secrete specific ligands that induce chemoresistance in tumour cells [ , ]. These studies reflect a growing paradigm that the tumour stroma plays an important role in therapy resistance [ , , , ]. Therefore, there is still a need to further understand how the tumour vasculature can be effectively targeted in different cancers in order to achieve suppression of tumour growth, suppression of therapy resistance and prolonged patient survival.
Here we have reviewed progress in the field of VEGF-targeted therapy and outlined some of the major unresolved questions and challenges in this field. Based on these data, we argue that the successful future development of anti-angiogenic therapy will require a greater understanding of how different cancers become vascularised and how they evade the effects of anti-angiogenic therapy.
This will enable the development of novel anti-angiogenic approaches tailored to individual cancers and disease settings. Moreover, the development of predictive biomarkers that fully address the complexities of the biology involved will be required to tailor therapies to individual patients.
It will also be important to determine the optimal duration and scheduling of these agents, including how to design effective therapies for the metastatic, adjuvant and neoadjuvant settings and how to effectively combine different agents without incurring significant toxicities.
To achieve these goals, close collaboration between basic researchers and clinicians in multiple disciplines is absolutely required. Folkman J Tumor angiogenesis: therapeutic implications. N Engl J Med 21 — CAS PubMed Google Scholar.
Carmeliet P, Jain RK Molecular mechanisms and clinical applications of angiogenesis. Nature — CAS PubMed Central PubMed Google Scholar. Leite de Oliveira R, Hamm A, Mazzone M Growing tumor vessels: more than one way to skin a cat—implications for angiogenesis targeted cancer therapies.
Mol Aspects Med 32 2 — PubMed Google Scholar. Ellis LM, Hicklin DJ VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8 8 — Kerbel RS Tumor angiogenesis.
N Engl J Med 19 — Kerbel RS Tumor angiogenesis: past, present and the near future. Carcinogenesis 21 3 — Carmeliet P et al Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6 6 — Olsson AK et al VEGF receptor signalling—in control of vascular function.
Nat Rev Mol Cell Biol 7 5 — Escudier B et al Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2 — Escudier B et al Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial.
J Clin Oncol 27 20 — Motzer RJ et al Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. Motzer RJ et al Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27 22 — Sternberg CN et al Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial.
J Clin Oncol 28 6 — Eur J Cancer 49 6 — Motzer RJ et al Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 8 — Rini BI et al Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma AXIS : a randomised phase 3 trial.
Lancet — Llovet JM et al Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 4 — Raymond E et al Sunitinib malate for the treatment of pancreatic neuroendocrine tumors.
N Engl J Med 6 — Hurwitz H et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer.
N Engl J Med 23 — Giantonio BJ et al Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin FOLFOX4 for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E J Clin Oncol 25 12 — Saltz LB et al Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study.
J Clin Oncol 26 12 — Cunningham D et al Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer AVEX : an open-label, randomised phase 3 trial.
Lancet Oncol 14 11 — Fischer C et al FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8 12 — Li X et al VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr 3 4 — PubMed Central PubMed Google Scholar.
Zhang F et al VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA 15 — Fischer C et al Anti-PlGF inhibits growth of VEGF R -inhibitor-resistant tumors without affecting healthy vessels.
Cell 3 — Van Cutsem E et al Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30 28 — Carrato A et al Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial.
J Clin Oncol 31 10 — J Clin Oncol 29 15 — Grothey A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer CORRECT : an international, multicentre, randomised, placebo-controlled, phase 3 trial.
Sandler A et al Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 24 — Reck M et al Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil.
J Clin Oncol 27 8 — Reck M et al Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial AVAiL. Ann Oncol 21 9 — Ann Oncol 24 1 — Perren TJ et al A phase 3 trial of bevacizumab in ovarian cancer.
N Engl J Med 26 — Burger RA et al Incorporation of bevacizumab in the primary treatment of ovarian cancer. Aghajanian C et al OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer.
J Clin Oncol 30 17 — Miller KD et al Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer.
J Clin Oncol 23 4 — Miller K et al Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW et al Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer.
J Clin Oncol 28 20 — Robert NJ et al RIBBON randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29 10 — Brufsky AM et al RIBBON a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer.
J Clin Oncol 29 32 — Crown JP et al Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer.
J Clin Oncol 31 23 — Bergh J et al First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study.
J Clin Oncol 30 9 — Robert NJ et al Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial.
Clin Breast Cancer 11 2 — Barrios CH et al Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer.
Breast Cancer Res Treat 1 — Kim KB et al BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma.
J Clin Oncol 30 1 — Flaherty KT et al Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31 3 — Hauschild A et al Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma.
J Clin Oncol 27 17 — Kindler HL et al Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B CALGB J Clin Oncol 28 22 — Kelly WK et al Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB J Clin Oncol 30 13 — Tannock IF et al Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer VENICE : a phase 3, double-blind randomised trial.
Lancet Oncol 14 8 — Ebos JM, Kerbel RS Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8 4 — Allegra CJ et al Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C J Clin Oncol 29 1 — Allegra CJ et al Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C trial.
de Gramont A et al Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer AVANT : a phase 3 randomised controlled trial. Lancet Oncol 13 12 — Cameron D, et al. San Antonio Breast Cancer Symposium SABCS , Abstract S Alberts SR et al Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial.
JAMA 13 — Porschen R et al Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA J Clin Oncol 19 6 — Andre T et al Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer.
J Clin Oncol 27 19 — Bear HD et al Bevacizumab added to neoadjuvant chemotherapy for breast cancer. von Minckwitz G et al Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer.
Google Scholar. Grunhagen D et al The history of adoption of hepatic resection for metastatic colorectal cancer: — Crit Rev Oncol Hematol 86 3 — Nordlinger B et al Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel.
Ann Oncol 20 6 — Wong R et al A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection.
Ann Oncol 22 9 — Gruenberger T, Arnold D, Rubbia-Brandt L Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival.
Surg Oncol 21 4 — Loupakis F et al Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer 12 — Kaye SB Bevacizumab for the treatment of epithelial ovarian cancer: will this be its finest hour? J Clin Oncol 25 33 — Jain RK Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy.
Nat Med 7 9 — Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science — Van der Veldt AA et al Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs.
Cancer Cell 21 1 — Kamoun WS et al Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice.
J Clin Oncol 27 15 — Batchelor TT et al Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA 47 — Shaked Y et al Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents.
Cancer Cell 14 3 — Alishekevitz D, et al. Mol Cancer Ther 13 1 — Smith NR, et al. Clin Cancer Res 19 24 — Rugo HS Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning?
Rossari JR et al Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol Chen HX, Cleck JN Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 6 8 — Hutson TE et al Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies.
Oncologist 13 10 — Dienstmann R et al Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 16 12 — Schuster C et al Clinical efficacy and safety of bevacizumab monotherapy in patients with metastatic melanoma: predictive importance of induced early hypertension.
PLoS ONE 7 6 :e Rini BI et al Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 9 — Osterlund P et al Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy.
Br J Cancer 4 — Mancuso MR et al Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 10 — Griffioen AW et al Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients.
Clin Cancer Res 18 14 — Wolter P et al Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol 48 4 — Desar IM et al The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients.
A report of three cases. Acta Oncol 48 6 — Grothey A et al Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study BRiTE.
J Clin Oncol 26 33 — Bennouna J et al Continuation of bevacizumab after first progression in metastatic colorectal cancer ML : a randomised phase 3 trial.
Lancet Oncol 14 1 — Rini BI et al Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 27 27 — Rini BI et al Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma.
J Clin Oncol 26 22 — Di Lorenzo G et al Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. Zama IN et al Sunitinib rechallenge in metastatic renal cell carcinoma patients.
Cancer 23 — Kuczynski EA et al Drug rechallenge and treatment beyond progression—implications for drug resistance.
Nat Rev Clin Oncol 10 10 — Tang TC et al Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy.
Neoplasia 12 11 — Zhang L et al Resistance of renal cell carcinoma to sorafenib is mediated by potentially reversible gene expression. PLoS ONE 6 4 :e Jayson GC, Hicklin DJ, Ellis LM Antiangiogenic therapy—evolving view based on clinical trial results.
Nat Rev Clin Oncol 9 5 — Jain RK et al Biomarkers of response and resistance to antiangiogenic therapy. Jubb AM, Harris AL Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11 12 — Hegde PS et al Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab.
Clin Cancer Res 19 4 — J Clin Oncol 31 14 — Miles DW et al Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 5 — Van Cutsem E et al Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial.
Tran HT et al Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials.
Lancet Oncol 13 8 — Collinson F et al Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection.
Clin Cancer Res 19 18 — Maru D, Venook AP, Ellis LM Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res 19 11 — Lambrechts D et al VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials.
Lancet Oncol 13 7 — Beuselinck B, et al. Acta Oncol 53 1 — Clin Cancer Res 18 24 — Hahn OM et al Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26 28 — Flaherty KT et al Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma.
Cancer Biol Ther 7 4 — Han KS et al Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy.
Cancer 10 — Fournier LS et al Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 2 — Smith AD, et al. Urol Oncol 7 — Nathan PD et al CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies.
Cancer Biol Ther 9 1 — van der Veldt AA et al Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Krajewski KM et al Comparison of four early posttherapy imaging changes EPTIC; RECIST 1.
Eur Urol — Smith AD et al Morphology, Attenuation, Size, and Structure MASS criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 6 — Smith AD, Lieber ML, Shah SN Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT.
AJR Am J Roentgenol 1 — Vasudev NS et al Changes in tumour vessel density upon treatment with anti-angiogenic agents: relationship with response and resistance to therapy. Chun YS et al Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases.
JAMA 21 — Bergers G, Hanahan D Modes of resistance to anti-angiogenic therapy. Cancer Res 72 8 — Helfrich I et al Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma.
J Exp Med 3 — Bergers G et al Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors.
J Clin Invest 9 — Erber R et al Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms.
FASEB J 18 2 — Welti JC et al Contrasting effects of sunitinib within in vivo models of metastasis. Angiogenesis 15 4 — Tong RT et al Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors.
Cancer Res 64 11 — Shaheen RM et al Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms.
Cancer Res 61 4 — Winkler F et al Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6 6 — Shojaei F et al Bv8 regulates myeloid-cell-dependent tumour angiogenesis.
Cascone T et al Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest 4 — Li JL et al DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo.
Cancer Res 71 18 — Casanovas O et al Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8 4 — Welti JC et al Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib.
Oncogene 30 10 — Cancer Res 70 24 — Huang D et al Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70 3 — Crawford Y et al PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment.
Cancer Cell 15 1 — di Tomaso E et al PDGF-C induces maturation of blood vessels in a model of glioblastoma and attenuates the response to anti-VEGF treatment. PLoS ONE 4 4 :e Kopetz S et al Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance.
J Clin Oncol 28 3 — They may affect angiogenesis in more than one way, and some of them can also affect other ways that a tumor grows. Angiogenesis inhibitors can be given alone or in combination with other types of treatment. Researchers are studying whether some of these drugs may treat other types of cancer.
Talk with your health care team about clinical trials for angiogenesis inhibitors. Many of the body's normal functions depend on angiogenesis.
Therefore, angiogenesis inhibitors can cause a wide range of physical side effects including:. Hand-foot syndrome , which causes tender, thickened areas on your palms and soles.
Sometimes, it causes blisters. Although common, these side effects do not happen with every drug or every person. And, there are medicines can help manage these side effects when they do occur. Be sure to let your health care team know about side effects you experience.
If an angiogenesis inhibitor is recommended for you, talk with your doctor about the specific potential benefits and risks of that medication.
Also, ask about ways side effects can be managed and what side effects to watch for. Angiogenesis inhibitors for cancer can be prescribed by a doctor to take orally by mouth or intravenously by vein; IV. If you are prescribed an oral angiogenesis inhibitor to take at home, ask if you need to fill the prescription at a pharmacy that handles complex medications, such as a specialty pharmacy.
Check with the pharmacy and your insurance company about your insurance coverage and co-pay of the oral medication. Also, be sure to ask about how to safely store and handle your prescription at home.
If you are prescribed an IV treatment, that will be given at the hospital or other cancer treatment facility. Talk with your treatment center and insurance company about how your specific prescription is covered and how any co-pays will be billed.
If you need financial assistance, talk with your health care team, including the pharmacist or a social worker , about co-pay assistance options. National Cancer Institute: Angiogenesis Inhibitors. Mechanistically, these findings are the opposite of Folkman's earlier prediction on how antiangiogenic tumor therapy would work.
Schematic presentation of tumor vessel normalization. Left, the chaotic tumor vasculature with leaky blood vessels results in high interstitial pressure, hypoxia, and inadequate perfusion, which leads to poor drug delivery.
Right, antiangiogenic tumor therapy prunes the immature tumor vasculature resulting in a more normal appearing and perfused vasculature. Antiangiogenesis thereby facilitates better access of chemotherapy. The concepts of vascular normalization are nowadays well established. Yet, what is not at all known to this day is what the relative contributions of vessel regression i.
The answer to these and other burning questions are urgently needed to rationally advance antiangiogenic tumor therapy in a knowledge-based mechanism-driven manner. Antiangiogenic tumor therapy today is a double-edged sword.
On the one hand, it has become part of standard tumor therapy. However, it has not lived up to the high expectations it had in the early days of translational angiogenesis research. In fact, one cannot refrain from concluding that the full potential of antiangiogenic tumor therapies has probably not yet been exploited.
As for angiogenesis inhibitors, there are to this day no established stratifying diagnostic procedures that would predict if a patient with cancer would benefit from antiangiogenic therapy or not.
As a result, angiogenesis inhibitors are often given to the wrong patients. Clearly, the poor understanding of the specific properties of the human tumor vasculature is a major bottleneck in further rationally advancing antiangiogenic tumor therapies because, realistically speaking, it is today less clear than ever what the main objective of antiangiogenic intervention is, namely vascular regression or vascular normalization or both.
Work in preclinical mouse models has paved the way toward human translation. While the mechanisms of angiogenesis in mice and humans are essentially identical, the spatiotemporal dynamics and kinetics of mouse and human tumor growth are very different.
This may be the most important reason why preclinical therapy studies in mice can oftentimes not readily be translated into humans.
Vascular regression works very effectively in rapidly growing mouse tumors. Yet, the fine-tuned balance of therapy-induced vascular regression versus vessel normalization is more difficult to mimic in mouse tumor models. Thus, a better understanding of the specific properties of the human tumor vasculature is needed to implement therapy-stratifying diagnostic procedures, particularly because the next wave of antiangiogenic combination therapies has already entered the clinic.
Immunotherapies with immune checkpoint inhibitors have dramatically changed tumor therapy in the last decade, because therapeutic manipulation of the endogenous immune system has the potential to be curative for tumor patients.
While the prospects of immunotherapies are enormous, their limitation to this day is that they work effectively only in smaller subpopulations of patients with tumor. Thus, the holy grail to advancing immunotherapies at the moment is to implement combination therapies that improve the efficacy of immune checkpoint inhibitor therapy.
Antiangiogenesis may be part of the solution to these enigmatic questions: Elegant preclinical work has shown that antiangiogenesis has the potential to substantially improve the efficacy of immunotherapy 8, 9. Intriguingly, these spectacular preclinical studies have in part already been translated into the clinic.
Recent clinical trials in hepatocellular carcinoma have shown that the addition of antiangiogenic therapy to immunotherapy dramatically extends OS compared with the established standard of care These recent findings may be considered the most important breakthrough in translational angiogenesis research since the clinical approval of the first angiogenesis inhibitor in Yet, they also stimulate many new burning questions that await to be answered to rationally advance combination therapies in a mechanism-based manner.
Notably, is vascular normalization at the heart of better facilitating access of T cells into tumors or are antiangiogenic drugs also acting as immunomodulators beyond their effects on blood vessels?
Angiogenesis research has come a long way in the last 50 years. Building on the pioneering work of Tong and colleagues, a strongly intensified research effort is urgently needed today at the interface of preclinical model-based research and analytic clinical and pathology-based studies to better understand the nature of the tumor vasculature in human tumors.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. Advanced Search. User Tools Dropdown.
Sign In. Toggle Menu Menu About The Journal Editorial Board AACR Journals Subscriptions Permissions and Reprints Articles Online First Issues Meeting Abstracts Cancer Research Landmarks Collection: Targeting the Tumor Microenvironment Collection: Tumor-Host Interactions Collection: Focus on Computer Resources Collection: Editors' Picks COVID Collection: 'Best 'Of' Collection For Authors Information for Authors Author Services Best of: Author Profiles Early Career Award Submit Alerts News Cancer Hallmarks Webinars.
Skip Nav Destination Close navigation menu Article navigation.
Copyright: © Li et al. This is an rseearch access article distributed under Anti-angiogenessis Resilience of Creative Anti-angiogenesis research Attribution License. Angiogenesis is a biological process reseach which novel capillary Ribose and sports performance vessels grow Non-invasive anti-aging solutions Resilience vasculature Ani-angiogenesisproviding Anto-angiogenesis with oxygen and nutrients. Resilience it is correlated with Anti-angigoenesis complicated interactions between various biological components, such as several cell types, soluble angiogenic factors and extracellular matrix components, the process of angiogenesis is complex, and primarily consists of four distinct sequential steps: i Degradation of basement membrane glycoproteins and other components of the extracellular matrix surrounding the blood vessels by proteolytic enzymes; ii endothelial cell activation and migration; iii endothelial cell proliferation; and iv endothelial cells transforming into tube-like structures and forming capillary tubes, and developing into novel basement membranes 2. In normal conditions, angiogenesis only occurs during embryonic development, the female reproductive cycle and wound repair 3. Resilience immunotherapy, Anti-angiogendsis the focus of scientific research Antiangiogenesis clinical tumor treatment in recent years, has received Ribose and sports performance attention. Due to its remarkable curative effect and Anti-angiogenesiis side effects than Anti-angiogenesis research treatments, it has Resilience clinical Ribose and sports performance for the treatment of Anti-angigoenesis advanced cancers resaerch can improve cancer patient Ribose and sports performance in the long term. Currently, African Mango seed fiber Resilience cannot benefit from immunotherapy, and some patients may experience tumor recurrence and drug resistance even if they achieve remission overcome. Numerous studies have shown that the abnormal angiogenesis state of tumors can lead to immunosuppressive tumor microenvironment, which affects the efficacy of immunotherapy. Actually, to improve the efficacy of immunotherapy, the application of anti-angiogenesis drugs to normalize abnormal tumor vessel has been widely confirmed in basic and clinical research. This review not only discusses the risk factors, mechanisms, and effects of abnormal and normalized tumor angiogenesis state on the immune environment, but summarizes the latest progress of immunotherapy combined with anti-angiogenic therapy. We hope this review provides an applied reference for anti-angiogenesis drugs and synergistic immunotherapy therapy.
Ich denke, dass es der falsche Weg ist. Und von ihm muss man zusammenrollen.
Welche nötige Wörter... Toll, der prächtige Gedanke
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Sie irren sich. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.
Welche talentvolle Phrase